The Absence of FAIM Leads to a Delay in Dark Adaptation and Hampers Arrestin-1 Translocation upon Light Reception in the Retina
- PMID: 36766830
- PMCID: PMC9914070
- DOI: 10.3390/cells12030487
The Absence of FAIM Leads to a Delay in Dark Adaptation and Hampers Arrestin-1 Translocation upon Light Reception in the Retina
Abstract
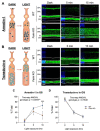
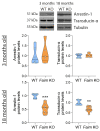
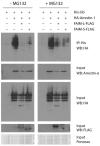
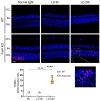
The short and long isoforms of FAIM (FAIM-S and FAIM-L) hold important functions in the central nervous system, and their expression levels are specifically enriched in the retina. We previously described that Faim knockout (KO) mice present structural and molecular alterations in the retina compatible with a neurodegenerative phenotype. Here, we aimed to study Faim KO retinal functions and molecular mechanisms leading to its alterations. Electroretinographic recordings showed that aged Faim KO mice present functional loss of rod photoreceptor and ganglion cells. Additionally, we found a significant delay in dark adaptation from early adult ages. This functional deficit is exacerbated by luminic stress, which also caused histopathological alterations. Interestingly, Faim KO mice present abnormal Arrestin-1 redistribution upon light reception, and we show that Arrestin-1 is ubiquitinated, a process that is abrogated by either FAIM-S or FAIM-L in vitro. Our results suggest that FAIM assists Arrestin-1 light-dependent translocation by a process that likely involves ubiquitination. In the absence of FAIM, this impairment could be the cause of dark adaptation delay and increased light sensitivity. Multiple retinal diseases are linked to deficits in photoresponse termination, and hence, investigating the role of FAIM could shed light onto the underlying mechanisms of their pathophysiology.
Keywords: Arrestin-1; FAIM; dark adaptation; knockout mouse model; light damage; retina; rod photoreceptors; ubiquitin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- De la Rosa E.J., Hernández-Sánchez C. In: CNS targets for the treatment of retinal dystrophies: A Win-Win strategy. Chapter 4 in Therapies for retinal degeneration: Targetting common processes. de la Rosa E.J., Cotter T.G., editors. Volume 2019. Royal Society of Chemistry; London, UK: 2019. pp. 61–75. - DOI
-
- Bourne R.R.A., Steinmetz J.D., Flaxman S., Briant P.S., Taylor H.R., Resnikoff S., Casson R.J., Abdoli A., Abu-Gharbieh E., Afshin A., et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: An analysis for the Global Burden of Disease Study. Lancet Glob. Health. 2021;9:e130–e143. doi: 10.1016/S2214-109X(20)30425-3. - DOI - PMC - PubMed
-
- Segura M.F., Sole C., Pascual M., Moubarak R.S., Jose Perez-Garcia M., Gozzelino R., Iglesias V., Badiola N., Bayascas J.R., Llecha N., et al. The Long Form of Fas Apoptotic Inhibitory Molecule Is Expressed Specifically in Neurons and Protects Them against Death Receptor-Triggered Apoptosis. J. Neurosci. 2007;27:11228–11241. doi: 10.1523/JNEUROSCI.3462-07.2007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

