Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood-Brain Barrier Model and Entry to Human Brain Organoids
- PMID: 36766845
- PMCID: PMC9914642
- DOI: 10.3390/cells12030503
Targeting Human Endothelial Cells with Glutathione and Alanine Increases the Crossing of a Polypeptide Nanocarrier through a Blood-Brain Barrier Model and Entry to Human Brain Organoids
Abstract
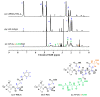
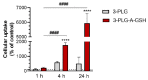
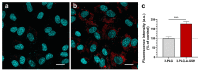
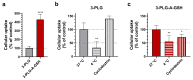
Nanoparticles (NPs) are the focus of research efforts that aim to develop successful drug delivery systems for the brain. Polypeptide nanocarriers are versatile platforms and combine high functionality with good biocompatibility and biodegradability. The key to the efficient brain delivery of NPs is the specific targeting of cerebral endothelial cells that form the blood-brain barrier (BBB). We have previously discovered that the combination of two different ligands of BBB nutrient transporters, alanine and glutathione, increases the permeability of vesicular NPs across the BBB. Our aim here was to investigate whether the combination of these molecules can also promote the efficient transfer of 3-armed poly(l-glutamic acid) NPs across a human endothelial cell and brain pericyte BBB co-culture model. Alanine and glutathione dual-targeted polypeptide NPs showed good cytocompatibility and elevated cellular uptake in a time-dependent and active manner. Targeted NPs had a higher permeability across the BBB model and could subsequently enter midbrain-like organoids derived from healthy and Parkinson's disease patient-specific stem cells. These results indicate that poly(l-glutamic acid) NPs can be used as nanocarriers for nervous system application and that the right combination of molecules that target cerebral endothelial cells, in this case alanine and glutathione, can facilitate drug delivery to the brain.
Keywords: 3-armed polypeptides; alanine; blood–brain barrier; brain endothelial cells; brain organoid; dual-targeting; glutathione; peptide nanocarriers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

