Exploring the Interactions of Oncolytic Viral Therapy and Immunotherapy of Anti-CTLA-4 for Malignant Melanoma Mice Model
- PMID: 36766849
- PMCID: PMC9914370
- DOI: 10.3390/cells12030507
Exploring the Interactions of Oncolytic Viral Therapy and Immunotherapy of Anti-CTLA-4 for Malignant Melanoma Mice Model
Abstract
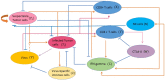
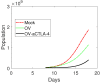
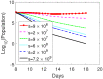
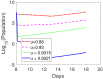
Oncolytic ability to direct target and lyse tumor cells makes oncolytic virus therapy (OVT) a promising approach to treating cancer. Despite its therapeutic potential to stimulate anti-tumor immune responses, it also has immunosuppressive effects. The efficacy of OVTs as monotherapies can be enhanced by appropriate adjuvant therapy such as anti-CTLA-4. In this paper, we propose a mathematical model to explore the interactions of combined therapy of oncolytic viruses and a checkpoint inhibitor, anti-CTLA-4. The model incorporates both the susceptible and infected tumor populations, natural killer cell population, virus population, tumor-specific immune populations, virus-specific immune populations, tumor suppressive cytokine IFN-g, and the effect of immune checkpoint inhibitor CTLA-4. In particular, we distinguish the tumor-specific immune abilities of CD8+ T, NK cells, and CD4+ T cells and describe the destructive ability of cytokine on tumor cells as well as the inhibitory capacity of CTLA-4 on various components. Our model is validated through the experimental results. We also investigate various dosing strategies to improve treatment outcomes. Our study reveals that tumor killing rate by cytokines, cytokine decay rate, and tumor growth rate play important roles on both the OVT monotherapy and the combination therapy. Moreover, parameters related to CD8+ T cell killing have a large impact on treatment outcomes with OVT alone, whereas parameters associated with IFN-g strongly influence treatment responses for the combined therapy. We also found that virus killing by NK cells may halt the desired spread of OVs and enhance the probability of tumor escape during the treatment. Our study reveals that it is the activation of host anti-tumor immune system responses rather than its direct destruction of the tumor cells plays a major biological function of the combined therapy.
Keywords: cytokines; immune checkpoint CTLA-4; mathematical modeling; melanoma; oncolytic virus therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

