Proteins Adsorbed during Intraoperative Hemoadsorption and Their In Vitro Effects on Endothelium
- PMID: 36766885
- PMCID: PMC9914797
- DOI: 10.3390/healthcare11030310
Proteins Adsorbed during Intraoperative Hemoadsorption and Their In Vitro Effects on Endothelium
Abstract
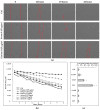
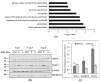
(1) Background: Hemoadsorption is a method of blood purification with a wide spectrum of indications. Pre-emptive use of hemoadsorption in patients undergoing heart surgery with cardiopulmonary bypass is considered to reduce the risk of postoperative systemic inflammatory response syndrome. The current study aimed to identify the spectrum of blood proteins adsorbed on the polymer matrix of the CytoSorb hemoadsorption system and to investigate their influence on cultured endothelial cells in vitro. (2) Methods: Adsorbers used for intraoperative hemoadsorption were obtained from patients undergoing on-pump valve surgery in acute endocarditis. Proteins were extracted from the adsorbers, purified, identified with mass-spectrometry and applied to cultured human aortic endothelial cells. (3) Results: A broad range of blood proteins were identified in the material eluted from the CytoSorb adsorber. When added to cultured ECs, these protein extracts caused severe reduction in cell viability and migration. After 24 h exposure, transcriptional changes with up-regulation of multiple metabolic regulators were observed and verified on the protein level. Genes responsible for control of mitosis were significantly down-regulated. (4) Conclusions: In summary, our data reveal that intraoperative hemoadsorption allows broad spectrum removal of a wide range of molecules eliciting endothelial damage.
Keywords: blood purification; endothelium; hemoadsorption; systemic inflammatory response.
Conflict of interest statement
V.P. and A.S. have received a research funding grant from CytoSorbents. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Squiccimarro E., Labriola C., Malvindi P.G., Margari V., Guida P., Visicchio G., Kounakis G., Favale A., Dambruoso P., Mastrototaro G., et al. Prevalence and Clinical Impact of Systemic Inflammatory Reaction after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019;33:1682–1690. doi: 10.1053/j.jvca.2019.01.043. - DOI - PubMed
-
- Vermeulen Windsant I.C., de Wit N.C.J., Sertorio J.T.C., van Bijnen A.A., Ganushchak Y.M., Heijmans J.H., Tanus-Santos J.E., Jacobs M.J., Maessen J.G., Buurman W.A. Hemolysis during cardiac surgery is associated with increased intravascular nitric oxide consumption and perioperative kidney and intestinal tissue damage. Front. Physiol. 2014;5:340. doi: 10.3389/fphys.2014.00340. - DOI - PMC - PubMed
-
- Giacinto O., Satriano U., Nenna A., Spadaccio C., Lusini M., Mastroianni C., Nappi F., Chello M. Inflammatory Response and Endothelial Dysfunction Following Cardiopulmonary Bypass: Pathophysiology and Pharmacological Targets. Recent Pat. Inflamm. Allergy Drug Discov. 2019;13:158–173. doi: 10.2174/1872213X13666190724112644. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

