The Telomere-Telomerase System Is Detrimental to Health at High-Altitude
- PMID: 36767300
- PMCID: PMC9915065
- DOI: 10.3390/ijerph20031935
The Telomere-Telomerase System Is Detrimental to Health at High-Altitude
Abstract
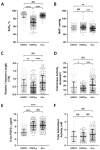
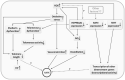
The hypobaric-hypoxia environment at high-altitude (HA, >2500 m) may influence DNA damage due to the production of reactive molecular species and high UV radiation. The telomere system, vital to chromosomal integrity and cellular viability, is prone to oxidative damages contributing to the severity of high-altitude disorders such as high-altitude pulmonary edema (HAPE). However, at the same time, it is suggested to sustain physical performance. This case-control study, comprising 210 HAPE-free (HAPE-f) sojourners, 183 HAPE-patients (HAPE-p) and 200 healthy highland natives (HLs) residing at ~3500 m, investigated telomere length, telomerase activity, and oxidative stress biomarkers. Fluidigm SNP genotyping screened 65 single nucleotide polymorphisms (SNPs) in 11 telomere-maintaining genes. Significance was attained at p ≤ 0.05 after adjusting for confounders and correction for multiple comparisons. Shorter telomere length, decreased telomerase activity and increased oxidative stress were observed in HAPE patients; contrarily, longer telomere length and elevated telomerase activity were observed in healthy HA natives compared to HAPE-f. Four SNPs and three haplotypes are associated with HAPE, whereas eight SNPs and nine haplotypes are associated with HA adaptation. Various gene-gene interactions and correlations between/among clinical parameters and biomarkers suggested the presence of a complex interplay underlining HAPE and HA adaptation physiology. A distinctive contribution of the telomere-telomerase system contributing to HA physiology is evident in this study. A normal telomere system may be advantageous in endurance training.
Keywords: adaptation; genetic predisposition; high-altitude; high-altitude pulmonary edema; telomerase; telomere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

