Degradation Mechanisms of 4,7-Dihydroxycoumarin Derivatives in Advanced Oxidation Processes: Experimental and Kinetic DFT Study
- PMID: 36767412
- PMCID: PMC9916318
- DOI: 10.3390/ijerph20032046
Degradation Mechanisms of 4,7-Dihydroxycoumarin Derivatives in Advanced Oxidation Processes: Experimental and Kinetic DFT Study
Abstract
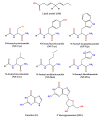
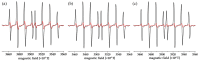
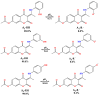
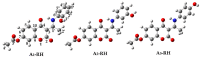
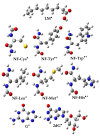
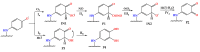
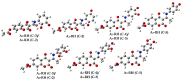
Coumarins represent a broad class of compounds with pronounced pharmacological properties and therapeutic potential. The pursuit of the commercialization of these compounds requires the establishment of controlled and highly efficient degradation processes, such as advanced oxidation processes (AOPs). Application of this methodology necessitates a comprehensive understanding of the degradation mechanisms of these compounds. For this reason, possible reaction routes between HO• and recently synthesized aminophenol 4,7-dihydroxycoumarin derivatives, as model systems, were examined using electron paramagnetic resonance (EPR) spectroscopy and a quantum mechanical approach (a QM-ORSA methodology) based on density functional theory (DFT). The EPR results indicated that all compounds had significantly reduced amounts of HO• radicals present in the reaction system under physiological conditions. The kinetic DFT study showed that all investigated compounds reacted with HO• via HAT/PCET and SPLET mechanisms. The estimated overall rate constants (koverall) correlated with the EPR results satisfactorily. Unlike HO• radicals, the newly formed radicals did not show (or showed negligible) activity towards biomolecule models representing biological targets. Inactivation of the formed radical species through the synergistic action of O2/NOx or the subsequent reaction with HO• was thermodynamically favored. The ecotoxicity assessment of the starting compounds and oxidation products, formed in multistage reactions with O2/NOx and HO•, indicated that the formed products showed lower acute and chronic toxicity effects on aquatic organisms than the starting compounds, which is a prerequisite for the application of AOPs procedures in the degradation of compounds.
Keywords: 4,7-dihydroxycoumarin; AOPs; DFT; EPR; QM−ORSA; hydroxyl radical; radical scavenging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Al-Majedy Y.K., Kadhum A.A.H., Al-Amiery A.A., Mohamad A.B. Coumarins: The antimicrobial agents. Sys. Rev. Pharm. 2017;8:62. doi: 10.5530/srp.2017.1.11. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

