Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families
- PMID: 36767903
- PMCID: PMC9915936
- DOI: 10.3390/ijerph20032542
Birth Outcomes in DES Children and Grandchildren: A Multigenerational National Cohort Study on Informative Families
Abstract
Objective: Diethylstilbestrol (DES), a potent synthetic nonsteroidal estrogen belonging to the family of endocrine disrupting chemicals (EDCs), can cross the placenta and may cause permanent adverse health effects in the exposed mothers, their children (exposed in utero), and also their grandchildren through germline contribution to the zygote. This study evaluated pregnancy duration and birthweight (BW) variations in the children and grandchildren born before, during, and after maternal DES treatment in the same informative families, to rule out genetic, endocrine, and environmental factors.
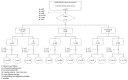
Design and setting: Nationwide retrospective observational study on 529 families of DES-treated women registered at the HHORAGES-France Association. The inclusion criteria were: (i) women with at least three pregnancies and three viable children among whom the first was not exposed in utero to DES, followed by one or more children with fetal exposure to DES, and then by one or more children born after DES treatment; (ii) women with at least one pre-DES or post-DES grandchild and one DES grandchild; (iii) confirmed data on total DES dose. Women with severe pathologies or whose illness status, habitat, lifestyle habits, profession, treatment changed between pregnancies, and all mothers who reported pregnancy-related problems, were excluded.
Results: In all, 74 women met all criteria. The preterm birth (PTB) rate was 2.7% in pre-DES, 14.9% in DES, and 10.8% in post-DES children (Cochran-Armitage test for trend, p = 0.0095). The mean BW was higher in DES than pre-DES full-term neonates (≥37 weeks of gestation) (p = 0.007). In grandchildren, BW was not different, whereas the PTB and low BW rates were slightly increased in children of DES women.
Conclusions: These data within the same informative families show the DES impact on BW and PTB in DES and post-DES children and grandchildren. In particular, mean BW was higher in DES than pre-DES full-term neonates. This result may be in opposition to previous data from American cohorts, which reported lower BW in DES children, but is consistent with animal study. Our retrospective observational study highlights a multigenerational and likely transgenerational effect of this EDC in humans.
Keywords: birthweight; diethylstilbestrol (DES); endocrine-disrupting chemicals (EDC); epigenetic; multigenerational transmission; prenatal exposure.
Conflict of interest statement
Authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The HHORAGES-France Association is financed exclusively by subscriptions and donations. MOS-G is a researcher, president of the HHORAGES-France Association and a mother concerned with DES and other synthetic hormones. Note: The association HHORAGES-France, a patient family association, is registered at the Epidemiological Portal of French Health Databases from INSERM (French National Institute for Medical Research) and AVIESAN (National Alliance for Life Sciences and Health) (epidemiologiefrance.aviesan.fr).
Figures
References
-
- Miller R.K., Heckmann M.E., McKenzie R.C. Diethylstilbestrol: Placental Transfer, Metabolism, Covalent Binding and Fetal Distribution in the Wistar Rat. J. Pharmacol. Exp. Ther. 1982;220:358–365. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous