A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications
- PMID: 36768144
- PMCID: PMC9915451
- DOI: 10.3390/ijms24031821
A Newly Developed Chemically Defined Serum-Free Medium Suitable for Human Primary Keratinocyte Culture and Tissue Engineering Applications
Abstract
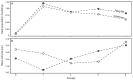
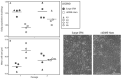
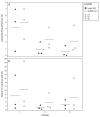
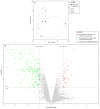
In our experience, keratinocytes cultured in feeder-free conditions and in commercially available defined and serum-free media cannot be as efficiently massively expanded as their counterparts grown in conventional bovine serum-containing medium, nor can they properly form a stratified epidermis in a skin substitute model. We thus tested a new chemically defined serum-free medium, which we developed for massive human primary keratinocyte expansion and skin substitute production. Our medium, named Surge Serum-Free Medium (Surge SFM), was developed to be used alongside a feeder layer. It supports the growth of keratinocytes freshly isolated from a skin biopsy and cryopreserved primary keratinocytes in cultured monolayers over multiple passages. We also show that keratin-19-positive epithelial stem cells are retained through serial passaging in Surge SFM cultures. Transcriptomic analyses suggest that gene expression is similar between keratinocytes cultured with either Surge SFM or the conventional serum-containing medium. Additionally, Surge SFM can be used to produce bilayered self-assembled skin substitutes histologically similar to those produced using serum-containing medium. Furthermore, these substitutes were grafted onto athymic mice and persisted for up to six months. In conclusion, our new chemically defined serum-free keratinocyte culture medium shows great promise for basic research and clinical applications.
Keywords: cell culture; defined medium; skin; stem cells; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
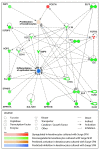
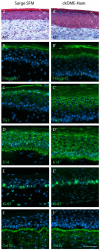
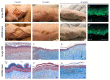
References
-
- Green H., Rheinwald J., Sun T. Properties of an epithelial cell type in culture: The epidermal keratinocyte and its dependence on products of the fibroblast. Prog. Clin. Biol. Res. 1977;17:493–500. - PubMed
-
- Cortez Ghio S., Cantin-Warren L., Guignard R., Larouche D., Germain L. Are the Effects of the Cholera Toxin and Isoproterenol on Human Keratinocytes’ Proliferative Potential Dependent on Whether They Are Co-Cultured with Human or Murine Fibroblast Feeder Layers? Int. J. Mol. Sci. 2018;19:2174. doi: 10.3390/ijms19082174. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

