HIV Replication Increases the Mitochondrial DNA Content of Plasma Extracellular Vesicles
- PMID: 36768245
- PMCID: PMC9916095
- DOI: 10.3390/ijms24031924
HIV Replication Increases the Mitochondrial DNA Content of Plasma Extracellular Vesicles
Abstract
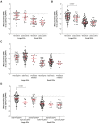
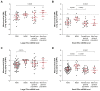
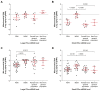
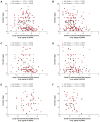
Extracellular vesicles (EVs) and their cargo have been studied intensively as potential sources of biomarkers in HIV infection; however, their DNA content, particularly the mitochondrial portion (mtDNA), remains largely unexplored. It is well known that human immunodeficiency virus (HIV) infection and prolonged antiretroviral therapy (ART) lead to mitochondrial dysfunction and reduced mtDNA copy in cells and tissues. Moreover, mtDNA is a well-known damage-associated molecular pattern molecule that could potentially contribute to increased immune activation, oxidative stress, and inflammatory response. We investigated the mtDNA content of large and small plasma EVs in persons living with HIV (PLWH) and its implications for viral replication, ART use, and immune status. Venous blood was collected from 196 PLWH, ART-treated or ART-naïve (66 with ongoing viral replication, ≥20 copies/mL), and from 53 HIV-negative persons, all recruited at five HIV testing or treatment centers in Burkina Faso. Large and small plasma EVs were purified and counted, and mtDNA level was measured by RT-qPCR. Regardless of HIV status, mtDNA was more abundant in large than small EVs. It was more abundant in EVs of viremic than aviremic and control participants and tended to be more abundant in participants treated with Tenofovir compared with Zidovudine. When ART treatment was longer than six months and viremia was undetectable, no variation in EV mtDNA content versus CD4 and CD8 count or CD4/CD8 ratio was observed. However, mtDNA in large and small EVs decreased with years of HIV infection and ART. Our results highlight the impact of viral replication and ART on large and small EVs' mtDNA content. The mechanisms underlying the differential incorporation of mtDNA into EVs and their effects on the surrounding cells warrant further investigation.
Keywords: HIV-1; antiretroviral therapy; extracellular vesicles; mitochondrial DNA; tenofovir; zidovudine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Hubert A., Subra C., Jenabian M.A., Tremblay Labrecque P.F., Tremblay C., Laffont B., Provost P., Routy J.P., Gilbert C. Elevated Abundance, Size, and MicroRNA Content of Plasma Extracellular Vesicles in Viremic HIV-1+ Patients: Correlations With Known Markers of Disease Progression. J. Acquir. Immune Defic. Syndr. 2015;70:219–227. doi: 10.1097/QAI.0000000000000756. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

