Coordinated Loss and Acquisition of NK Cell Surface Markers Accompanied by Generalized Cytokine Dysregulation in COVID-19
- PMID: 36768315
- PMCID: PMC9917026
- DOI: 10.3390/ijms24031996
Coordinated Loss and Acquisition of NK Cell Surface Markers Accompanied by Generalized Cytokine Dysregulation in COVID-19
Abstract
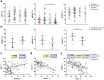
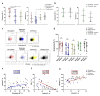
Coronavirus disease 2019 (COVID-19), caused by the SARS-CoV-2 virus, is accompanied by a dysregulated immune response. In particular, NK cells, involved in the antiviral response, are affected by the infection. This study aimed to investigate circulating NK cells with a focus on their activation, depletion, changes in the surface expression of key receptors, and functional activity during COVID-19, among intensive care unit (ICU) patients, moderately ill patients, and convalescents (CCP). Our data confirmed that NK cell activation in patients with COVID-19 is accompanied by changes in circulating cytokines. The progression of COVID-19 was associated with a coordinated decrease in the proportion of NKG2D+ and CD16+ NK cells, and an increase in PD-1, which indicated their exhaustion. A higher content of NKG2D+ NK cells distinguished surviving patients from non-survivors in the ICU group. NK cell exhaustion in ICU patients was additionally confirmed by a strong negative correlation of PD-1 and natural cytotoxicity levels. In moderately ill patients and convalescents, correlations were found between the levels of CD57, NKG2C, and NKp30, which may indicate the formation of adaptive NK cells. A reduced NKp30 level was observed in patients with a lethal outcome. Altogether, the phenotypic changes in circulating NK cells of COVID-19 patients suggest that the intense activation of NK cells during SARS-CoV-2 infection, most likely induced by cytokines, is accompanied by NK cell exhaustion, the extent of which may be critical for the disease outcome.
Keywords: COVID-19; NK cells; SARS-CoV-2; adaptive-like NK cells; coordinative response; cytokines; phenotype.
Conflict of interest statement
The authors declare the absence of obvious and potential conflicts of interest related to the publication of this article.
Figures


Similar articles
-
Distinct immunity dynamics of natural killer cells in mild and moderate COVID-19 cases during the Omicron variant phase.Front Immunol. 2025 May 12;16:1594296. doi: 10.3389/fimmu.2025.1594296. eCollection 2025. Front Immunol. 2025. PMID: 40421029 Free PMC article.
-
Elevated levels of cell-free NKG2D-ligands modulate NKG2D surface expression and compromise NK cell function in severe COVID-19 disease.Front Immunol. 2024 Feb 12;15:1273942. doi: 10.3389/fimmu.2024.1273942. eCollection 2024. Front Immunol. 2024. PMID: 38410511 Free PMC article.
-
SARS-CoV-2 infection impairs NK cell functions via activation of the LLT1-CD161 axis.Front Immunol. 2023 May 23;14:1123155. doi: 10.3389/fimmu.2023.1123155. eCollection 2023. Front Immunol. 2023. PMID: 37287972 Free PMC article.
-
Natural killer cell exhaustion in SARS-CoV-2 infection.Innate Immun. 2022 Aug;28(6):189-198. doi: 10.1177/17534259221077750. Epub 2022 Jun 22. Innate Immun. 2022. PMID: 35733383 Free PMC article. Review.
-
NK cells in COVID-19-from disease to vaccination.J Leukoc Biol. 2023 Oct 26;114(5):507-512. doi: 10.1093/jleuko/qiad031. J Leukoc Biol. 2023. PMID: 36976012 Review.
Cited by
-
HLA-DR Expression in Natural Killer Cells Marks Distinct Functional States, Depending on Cell Differentiation Stage.Int J Mol Sci. 2024 Apr 23;25(9):4609. doi: 10.3390/ijms25094609. Int J Mol Sci. 2024. PMID: 38731828 Free PMC article.
-
Alterations in the CD56- and CD56+ T Cell Subsets during COVID-19.Int J Mol Sci. 2023 May 20;24(10):9047. doi: 10.3390/ijms24109047. Int J Mol Sci. 2023. PMID: 37240393 Free PMC article.
-
Phenotypic Changes in T and NK Cells Induced by Sputnik V Vaccination.Vaccines (Basel). 2023 May 31;11(6):1047. doi: 10.3390/vaccines11061047. Vaccines (Basel). 2023. PMID: 37376436 Free PMC article.
-
Natural killer cell dysfunction is associated with colorectal cancer with severe COVID-19.World J Gastrointest Oncol. 2025 May 15;17(5):104591. doi: 10.4251/wjgo.v17.i5.104591. World J Gastrointest Oncol. 2025. PMID: 40487965 Free PMC article.
-
Innate immune response in COVID-19: single-cell multi-omics profile of NK lymphocytes in a clinical case series.Cell Commun Signal. 2024 Oct 15;22(1):496. doi: 10.1186/s12964-024-01867-5. Cell Commun Signal. 2024. PMID: 39407208 Free PMC article.
References
-
- COVID Live-Coronavirus Statistics–Worldometer. [(accessed on 22 April 2022)]. Available online: https://www.worldometers.info/coronavirus/
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

