A Cell-Based Assessment of the Muscle Anabolic Potential of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates
- PMID: 36768324
- PMCID: PMC9916327
- DOI: 10.3390/ijms24032001
A Cell-Based Assessment of the Muscle Anabolic Potential of Blue Whiting (Micromesistius poutassou) Protein Hydrolysates
Abstract
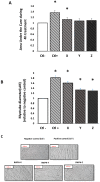
Blue whiting (BW) represents an underutilised fish species containing a high-quality protein and amino acid (AA) profile with numerous potentially bioactive peptide sequences, making BW an economic and sustainable alternative source of protein. This study investigated the impact of three different BW protein hydrolysates (BWPH-X, Y and Z) on growth, proliferation and muscle protein synthesis (MPS) in skeletal muscle (C2C12) myotubes. BWPHs were hydrolysed using different enzymatic and heat exposures and underwent simulated gastrointestinal digestion (SGID), each resulting in a high degree of hydrolysis (33.41-37.29%) and high quantities of low molecular mass peptides (86.17-97.12% <1 kDa). C2C12 myotubes were treated with 1 mg protein equivalent/mL of SGID-BWPHs for 4 h. Muscle growth and myotube thickness were analysed using an xCelligence™ platform. Anabolic signalling (phosphorylation of mTOR, rpS6 and 4E-BP1) and MPS measured by puromycin incorporation were assessed using immunoblotting. BWPH-X significantly increased muscle growth (p < 0.01) and myotube thickness (p < 0.0001) compared to the negative control (amino acid and serum free media). Muscle protein synthesis (MPS), as measured by puromycin incorporation, was significantly higher after incubation with BWPH-X compared with the negative control, but did not significantly change in response to BWPH-Y and Z treatments. Taken together, these preliminary findings demonstrate the anabolic potential of some but not all BWPHs on muscle enhancement, thus providing justification for human dietary intervention studies to confirm and translate the results of such investigations to dietary recommendations and practices.
Keywords: C2C12 cells; blue whiting protein hydrolysates; muscle growth; muscle protein synthesis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

