GBA1 Gene Mutations in α-Synucleinopathies-Molecular Mechanisms Underlying Pathology and Their Clinical Significance
- PMID: 36768367
- PMCID: PMC9917178
- DOI: 10.3390/ijms24032044
GBA1 Gene Mutations in α-Synucleinopathies-Molecular Mechanisms Underlying Pathology and Their Clinical Significance
Abstract
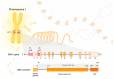
α-Synucleinopathies comprise a group of neurodegenerative diseases characterized by altered accumulation of a protein called α-synuclein inside neurons and glial cells. This aggregation leads to the formation of intraneuronal inclusions, Lewy bodies, that constitute the hallmark of α-synuclein pathology. The most prevalent α-synucleinopathies are Parkinson's disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). To date, only symptomatic treatment is available for these disorders, hence new approaches to their therapy are needed. It has been observed that GBA1 mutations are one of the most impactful risk factors for developing α-synucleinopathies such as PD and DLB. Mutations in the GBA1 gene, which encodes a lysosomal hydrolase β-glucocerebrosidase (GCase), cause a reduction in GCase activity and impaired α-synuclein metabolism. The most abundant GBA1 gene mutations are N370S or N409S, L444P/L483P and E326K/E365K. The mechanisms by which GCase impacts α-synuclein aggregation are poorly understood and need to be further investigated. Here, we discuss some of the potential interactions between α-synuclein and GCase and show how GBA1 mutations may impact the course of the most prevalent α-synucleinopathies.
Keywords: GBA1 mutations; Gaucher’s disease; Parkinson’s disease; dementia with Lewy bodies; glucocerebrosidase; glycosylceramidase; multiple system atrophy; α-Synuclein; α-synucleinopathies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

