Clinical Trials in Prader-Willi Syndrome: A Review
- PMID: 36768472
- PMCID: PMC9916985
- DOI: 10.3390/ijms24032150
Clinical Trials in Prader-Willi Syndrome: A Review
Abstract
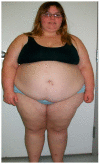
Prader-Willi syndrome (PWS) is a complex, genetic, neurodevelopmental disorder. PWS has three molecular genetic classes. The most common defect is due to a paternal 15q11-q13 deletion observed in about 60% of individuals. This is followed by maternal disomy 15 (both 15 s from the mother), found in approximately 35% of cases. the remaining individuals have a defect of the imprinting center that controls the activity of imprinted genes on chromosome 15. Mild cognitive impairment and behavior problems in PWS include self-injury, anxiety, compulsions, and outbursts in childhood, impacted by genetic subtypes. Food seeking and hyperphagia can lead to morbid obesity and contribute to diabetes and cardiovascular or orthopedic problems. The control of hyperphagia and improving food-related behaviors are the most important unmet needs in PWS and could be addressed with the development of a new therapeutic agent, as currently no approved therapeutics exist for PWS treatment. The status of clinical trials with existing results for the management of obesity and hyperphagia in PWS will be discussed in this review, including treatments such as beloranib, setmelanotide, a diazoxide choline controlled-release tablet (DCCR), an unacylated ghrelin analogue, oxytocin and related compounds, glucagon-like peptide 1 receptor agonists, surgical intervention, and transcranial direct-current stimulation.
Keywords: Prader–Willi syndrome; clinical trials; genetics; hyperphagia; obesity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Butler M.G., Hartin S.N., Hossain W.A., Manzardo A.M., Kimonis V., Dykens E., Gold J.A., Kim S.-J., Weisensel N., Tamura R., et al. Molecular Genetic Classification in Prader-Willi Syndrome: A Multisite Cohort Study. J. Med. Genet. 2018;56:149–153. doi: 10.1136/jmedgenet-2018-105301. - DOI - PMC - PubMed