Biomarkers of Aggressive Prostate Cancer at Diagnosis
- PMID: 36768533
- PMCID: PMC9916581
- DOI: 10.3390/ijms24032185
Biomarkers of Aggressive Prostate Cancer at Diagnosis
Abstract
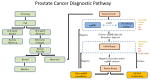
In the United States, prostate cancer (CaP) remains the second leading cause of cancer deaths in men. CaP is predominantly indolent at diagnosis, with a small fraction (25-30%) representing an aggressive subtype (Gleason score 7-10) that is prone to metastatic progression. This fact, coupled with the criticism surrounding the role of prostate specific antigen in prostate cancer screening, demonstrates the current need for a biomarker(s) that can identify clinically significant CaP and avoid unnecessary biopsy procedures and psychological implications of being diagnosed with low-risk prostate cancer. Although several diagnostic biomarkers are available to clinicians, very few comparative trials have been performed to assess the clinical effectiveness of these biomarkers. It is of note, however, that a majority of these clinical trials have been over-represented by men of Caucasian origin, despite the fact that African American men have a 1.7 times higher incidence and 2.1 times higher rate of mortality from prostate cancer. Biomarkers for CaP diagnosis based on the tissue of origin include urine-based gene expression assays (PCA3, Select MDx, ExoDx Prostate IntelliScore, Mi-Prostate Score, PCA3-PCGEM1 gene panel), blood-based protein biomarkers (4K, PHI), and tissue-based DNA biomarker (Confirm MDx). Another potential direction that has emerged to aid in the CaP diagnosis include multi-parametric magnetic resonance imaging (mpMRI) and bi-parametric magnetic resonance imaging (bpMRI), which in conjunction with clinically validated biomarkers may provide a better approach to predict clinically significant CaP at diagnosis. In this review, we discuss some of the adjunctive biomarker tests along with newer imaging modalities that are currently available to help clinicians decide which patients are at risk of having high-grade CaP on prostate biopsy with the emphasis on clinical utility of the tests across African American (AA) and Caucasian (CA) men.
Keywords: biomarkers; diagnosis; prostate cancer; race.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Negoita S., Feuer E.J., Mariotto A., Cronin K.A., Petkov V.I., Ms S.K.H., Benard V., Henley S.J., Anderson R.N., Fedewa S., et al. Annual Report to the Nation on the Status of Cancer, part II: Recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124:2801–2814. doi: 10.1002/cncr.31549. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

