Optimizing Axial and Peripheral Substitutions in Si-Centered Naphthalocyanine Dyes for Enhancing Aqueous Solubility and Photoacoustic Signal Intensity
- PMID: 36768560
- PMCID: PMC9916426
- DOI: 10.3390/ijms24032241
Optimizing Axial and Peripheral Substitutions in Si-Centered Naphthalocyanine Dyes for Enhancing Aqueous Solubility and Photoacoustic Signal Intensity
Abstract
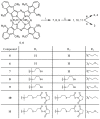
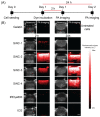
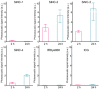
Photoacoustic imaging using external contrast agents is emerging as a powerful modality for real-time molecular imaging of deep-seated tumors. There are several chromophores, such as indocyanine green and IRDye800, that can potentially be used for photoacoustic imaging; however, their use is limited due to several drawbacks, particularly photostability. There is, therefore, an urgent need to design agents to enhance contrast in photoacoustic imaging. Naphthalocyanine dyes have been demonstrated for their use as photoacoustic contrast agents; however, their low solubility in aqueous solvents and high aggregation propensity limit their application. In this study, we report the synthesis and characterization of silicon-centered naphthalocyanine dyes with high aqueous solubility and near infra-red (NIR) absorption in the range of 850-920 nm which make them ideal candidates for photoacoustic imaging. A series of Silicon-centered naphthalocyanine dyes were developed with varying axial and peripheral substitutions, all in an attempt to enhance their aqueous solubility and improve photophysical properties. We demonstrate that axial incorporation of charged ammonium mesylate group enhances water solubility. Moreover, the incorporation of peripheral 2-methoxyethoxy groups at the α-position modulates the electronic properties by altering the π-electron delocalization and enhancing photoacoustic signal amplitude. In addition, all the dyes were synthesized to incorporate an N-hydroxysuccinimidyl group to enable further bioconjugation. In summary, we report the synthesis of water-soluble silicon-centered naphthalocyanine dyes with a high photoacoustic signal amplitude that can potentially be used as contrast agents for molecular photoacoustic imaging.
Keywords: image-guided therapy; naphthalocyanine dyes; photoacoustic imaging; silicon-centered naphthalocyanine dyes; water-soluble naphthalocyanines.
Conflict of interest statement
Scott Selfridge and Robert Pawle are full time employees of Akita Innovations, LLC, a provider of custom designed bioimaging dyes. All other authors declare no competing financial interest.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

