Comparative Efficacy of ALK Inhibitors for Treatment-Naïve ALK-Positive Advanced Non-Small Cell Lung Cancer with Central Nervous System Metastasis: A Network Meta-Analysis
- PMID: 36768562
- PMCID: PMC9917367
- DOI: 10.3390/ijms24032242
Comparative Efficacy of ALK Inhibitors for Treatment-Naïve ALK-Positive Advanced Non-Small Cell Lung Cancer with Central Nervous System Metastasis: A Network Meta-Analysis
Abstract
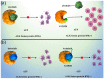
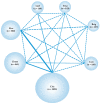
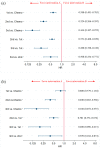
Central nervous system (CNS) metastases and acquired resistance complicate the treatment of anaplastic lymphoma kinase (ALK) rearrangement-positive (ALK-p) advanced non-small cell lung cancer (NSCLC). Thus, this review aimed to provide a comprehensive overview of brain metastasis, acquired resistance, and prospects for overcoming these challenges. A network meta-analysis of relevant phase III randomized controlled trials was performed to compare the efficacies of multiple ALK inhibitors by drug and generation in overall patients with ALK-p untreated advanced NSCLC and a subgroup of patients with CNS metastases. The primary endpoint was progression-free survival (PFS). Generation-specific comparison results showed that third-generation ALK inhibitors were significantly more effective than second-generation ALK inhibitors in prolonging the PFS of the subgroup of patients with CNS metastases. Drug-specific comparison results demonstrated that lorlatinib was the most effective in prolonging PFS, followed by brigatinib, alectinib, ensartinib, ceritinib, crizotinib, and chemotherapy. While lorlatinib was superior to brigatinib for PFS in the overall patient population, no significant difference between the two was found in the subgroup of patients with CNS metastases. These results can serve as a foundation for basic, clinical, and translational research and guide clinical oncologists in developing individualized treatment strategies for patients with ALK-p, ALK inhibitor-naive advanced NSCLC.
Keywords: ALK rearrangement; acquired resistance; brain metastasis; network meta-analysis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- The Japanese Lung Cancer Society Guideline. 2022. [(accessed on 3 December 2022)]. Available online: https://www.haigan.gr.jp/guideline/2022/
-
- Cognigni V., Pecci F., Lupi A., Pinterpe G., De Filippis C., Felicetti C., Cantini L., Berardi R. The landscape of ALK-rearranged non-small cell lung cancer: A comprehensive review of clinicopathologic, genomic characteristics, and therapeutic perspectives. Cancers. 2022;14:4765. doi: 10.3390/cancers14194765. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

