Advanced Glycation End Products Effects on Adipocyte Niche Stiffness and Cell Signaling
- PMID: 36768583
- PMCID: PMC9917270
- DOI: 10.3390/ijms24032261
Advanced Glycation End Products Effects on Adipocyte Niche Stiffness and Cell Signaling
Abstract
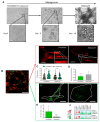
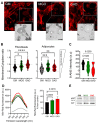
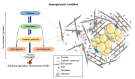
Adipose tissue metabolism under hyperglycemia results in Type II diabetes (T2D). To better understand how the adipocytes function, we used a cell culture that was exposed to glycation by adding intermediate carbonyl products, which caused chemical cross-linking and led to the formation of advanced glycation end products (AGEs). The AGEs increased the cells and their niche stiffness and altered the rheological viscoelastic properties of the cultured cells leading to altered cell signaling. The AGEs formed concomitant with changes in protein structure, quantified by spectroscopy using the 8-ANS and Nile red probes. The AGE effects on adipocyte differentiation were viewed by imaging and evidenced in a reduction in cellular motility and membrane dynamics. Importantly, the alteration led to reduced adipogenesis, that is also measured by qPCR for expression of adipogenic genes and cell signaling. The evidence of alteration in the plasma membrane (PM) dynamics (measured by CTxB binding and NP endocytosis), also led to the impairment of signal transduction and a decrease in AKT phosphorylation, which hindered downstream insulin signaling. The study, therefore, presents a new interpretation of how AGEs affect the cell niche, PM stiffness, and cell signaling leading to an impairment of insulin signaling.
Keywords: AGEs; adipocytes; adipogenesis; carbonyl compounds; cell migration; cytoskeleton reorganization; niche stiffness; plasma-membrane stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Briceno Noriega D., Zenker H.E., Croes C.A., Ewaz A., Ruinemans-Koerts J., Savelkoul H.F.J., van Neerven R.J.J., Teodorowicz M. Receptor Mediated Effects of Advanced Glycation End Products (AGEs) on Innate and Adaptative Immunity: Relevance for Food Allergy. Nutrients. 2022;14:371. doi: 10.3390/nu14020371. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

