The Gold(I) Complex with Plant Hormone Kinetin Shows Promising In Vitro Anticancer and PPARγ Properties
- PMID: 36768617
- PMCID: PMC9916778
- DOI: 10.3390/ijms24032293
The Gold(I) Complex with Plant Hormone Kinetin Shows Promising In Vitro Anticancer and PPARγ Properties
Abstract
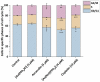
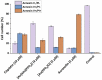
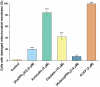
Motivated by the clinical success of gold(I) metallotherapeutic Auranofin in the effective treatment of both inflammatory and cancer diseases, we decided to prepare, characterize, and further study the [Au(kin)(PPh3)] complex (1), where Hkin = kinetin, 6-furfuryladenine, for its in vitro anti-cancer and anti-inflammatory activities. The results revealed that the complex (1) had significant in vitro cytotoxicity against human cancer cell lines (A2780, A2780R, PC-3, 22Rv1, and THP-1), with IC50 ≈ 1-5 μM, which was even significantly better than that for the conventional platinum-based drug Cisplatin while comparable with Auranofin. Although its ability to inhibit transcription factor NF-κB activity did not exceed the comparative drug Auranofin, it has been found that it is able to positively influence peroxisome-proliferator-activated receptor-gamma (PPARγ), and as a consequence of this to have the impact of moderating/reducing inflammation. The cellular effects of the complex (1) in A2780 cancer cells were also investigated by cell cycle analysis, induction of apoptosis, intracellular ROS production, activation of caspases 3/7 and disruption of mitochondrial membrane potential, and shotgun proteomic analysis. Proteomic analysis of R2780 cells treated with complex (1) and starting compounds revealed possible different places of the effect of the studied compounds. Moreover, the time-dependent cellular accumulation of copper was studied by means of the mass spectrometry study with the aim of exploring the possible mechanisms responsible for its biological effects.
Keywords: PPAR; ROS; anti-inflammatory; anticancer; apoptosis; cell cycle; gold(I) complex; in vitro; kinetin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

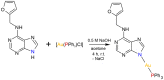







References
-
- Marzo T., Cirri D., Gabbiani C., Gamberi T., Magherini F., Pratesi A., Guerri A., Biver T., Binacchi F., Stefanini M., et al. Auranofin, Et3PAuCl, and Et3PAuI Are Highly Cytotoxic on Colorectal Cancer Cells: A Chemical and Biological Study. ACS Med. Chem. Lett. 2017;8:997–1001. doi: 10.1021/acsmedchemlett.7b00162. - DOI - PMC - PubMed
-
- Marzo T., Cirri D., Pollini S., Prato M., Fallani S., Cassetta M.I., Novelli A., Rossolini G.M., Messori L. Auranofin and its Analogues Show Potent Antimicrobial Activity against Multidrug-Resistant Pathogens: Structure–Activity Relationships. ChemMedChem. 2018;13:2448–2454. doi: 10.1002/cmdc.201800498. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

