NMR Study on Laccase Polymerization of Kraft Lignin Using Different Enzymes Source
- PMID: 36768678
- PMCID: PMC9917248
- DOI: 10.3390/ijms24032359
NMR Study on Laccase Polymerization of Kraft Lignin Using Different Enzymes Source
Abstract
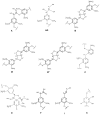
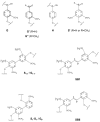
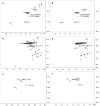
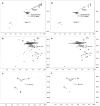
The usage of laccases is a sustainable and environmentally friendly approach to modifying the Kraft lignin structure for use in certain applications. However, the inherent structure of Kraft lignin, as well as that resulting from laccase modification, still presents challenges for fundamental comprehension and successful lignin valorization. In this study, bacterial and fungal laccases were employed to modify eucalypt Kraft lignin. To evaluate the type and range of the chemical and structural changes of laccase-treated lignins, different NMR techniques, including solution 1H and 2D NMR (heteronuclear single quantum correlation (HSQC)), and solid-state 13C NMR, were applied. Size exclusion chromatography and infrared spectroscopy were also used. Interestingly, HSQC analysis showed substantial changes in the oxygenated aliphatic region of lignins, showing an almost complete absence of signals corresponding to side-chains due to laccase depolymerization. Simultaneously, a significant loss of aromatic signals was observed by HSQC and 1H NMR, which was attributed to a deprotonation of the lignin benzenic rings due to polymerization/condensation by laccase reactions. Then, condensed structures, such as α-5', 5-5', and 4-O-5', were detected by HSQC and 13C NMR, supporting the increment in molecular weight, as well as the phenolic content reduction determined in lignins.
Keywords: Kraft lignin; NMR characterization; eucalypt; laccase; polymerization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- El Bouhali A., Gnanasekar P., Habibi Y. Lignin-Based Materials for Biomedical Applications. Elsevier; Amsterdam, The Netherlands: 2021. Chemical modifications of lignin; pp. 159–194.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

