Severe Asthma Remissions Induced by Biologics Targeting IL5/IL5r: Results from a Multicenter Real-Life Study
- PMID: 36768778
- PMCID: PMC9916787
- DOI: 10.3390/ijms24032455
Severe Asthma Remissions Induced by Biologics Targeting IL5/IL5r: Results from a Multicenter Real-Life Study
Abstract
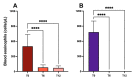
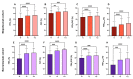
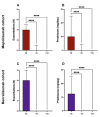
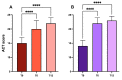
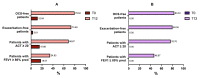
Add-on biological therapy has proven to be effective in many patients with severe eosinophilic asthma. In this observational multicenter retrospective study, we report the results obtained with mepolizumab and benralizumab in severe asthmatics treated for 12 months in a real-life setting. In these patients, peripheral eosinophil levels, pulmonary function trends, exacerbation rates, systemic corticosteroid use, and symptom control were evaluated during the observation period, to understand which patients met all the criteria in order to be considered in disease remission. The percentage of remittent patients was 30.12% in the mepolizumab-treated subgroup, while in the benralizumab-treated subgroup, patients in complete disease remission were 40%, after 12 months. The results of this study confirm the efficacy of anti-IL-5 biologic drugs in the treatment of severe eosinophilic asthma in a real-life setting.
Keywords: OCS-dependent asthma; airway inflammation; anti-IL 5; asthma remission; benralizumab; mepolizumab; severe asthma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- GINA Main Report. [(accessed on 14 December 2022)]. Available online: https://ginasthma.org/gina-reports/
-
- Soriano J.B., Abajobir A.A., Abate K.H., Abera S.F., Agrawal A., Ahmed M.B., Aichour A.N., Aichour I., Aichour M.T.E., Alam K., et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. - DOI - PMC - PubMed
-
- Bikov A., Oğuzülgen I.K., Baiardini I., Contoli M., Emelyanov A., Fassio O., Ivancevich J.C., Kaidashev I., Kowal K., Labor M., et al. Beliefs and preferences regarding biological treatments for severe asthma. World Allergy Organ. J. 2020;13:100441. doi: 10.1016/j.waojou.2020.100441. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

