LHC-like Proteins: The Guardians of Photosynthesis
- PMID: 36768826
- PMCID: PMC9916820
- DOI: 10.3390/ijms24032503
LHC-like Proteins: The Guardians of Photosynthesis
Abstract
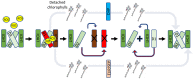
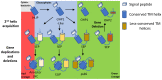
The emergence of chlorophyll-containing light-harvesting complexes (LHCs) was a crucial milestone in the evolution of photosynthetic eukaryotic organisms. Light-harvesting chlorophyll-binding proteins form complexes in proximity to the reaction centres of photosystems I and II and serve as an antenna, funnelling the harvested light energy towards the reaction centres, facilitating photochemical quenching, thereby optimizing photosynthesis. It is now generally accepted that the LHC proteins evolved from LHC-like proteins, a diverse family of proteins containing up to four transmembrane helices. Interestingly, LHC-like proteins do not participate in light harvesting to elevate photosynthesis activity under low light. Instead, they protect the photosystems by dissipating excess energy and taking part in non-photochemical quenching processes. Although there is evidence that LHC-like proteins are crucial factors of photoprotection, the roles of only a few of them, mainly the stress-related psbS and lhcSR, are well described. Here, we summarize the knowledge gained regarding the evolution and function of the various LHC-like proteins, with emphasis on those strongly related to photoprotection. We further suggest LHC-like proteins as candidates for improving photosynthesis in significant food crops and discuss future directions in their research.
Keywords: light-harvesting; non-photochemical quenching; photoinhibition; photoprotection; photosynthesis.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Levin G., Kulikovsky S., Liveanu V., Eichenbaum B., Meir A., Isaacson T., Tadmor Y., Adir N., Schuster G. The Desert Green Algae Chlorella Ohadii Thrives at Excessively High Light Intensities by Exceptionally Enhancing the Mechanisms That Protect Photosynthesis from Photoinhibition. Plant J. 2021;106:1260–1277. doi: 10.1111/tpj.15232. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

