NMDA Receptor and Its Emerging Role in Cancer
- PMID: 36768862
- PMCID: PMC9917092
- DOI: 10.3390/ijms24032540
NMDA Receptor and Its Emerging Role in Cancer
Abstract
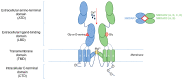
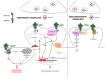
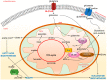
Glutamate is a key player in excitatory neurotransmission in the central nervous system (CNS). The N-methyl-D-aspartate receptor (NMDAR) is a glutamate-gated ion channel which presents several unique features and is involved in various physiological and pathological neuronal processes. Thanks to great efforts in neuroscience, its structure and the molecular mechanisms controlling its localization and functional regulation in neuronal cells are well known. The signaling mediated by NMDAR in neurons is very complex as it depends on its localization, composition, Ca2+ influx, and ion flow-independent conformational changes. Moreover, NMDA receptors are highly diffusive in the plasma membrane of neurons, where they form heterocomplexes with other membrane receptors and scaffold proteins which determine the receptor function and activation of downstream signaling. Interestingly, a recent paper demonstrates that NMDAR signaling is involved in epithelial cell competition, an evolutionary conserved cell fitness process influencing cancer initiation and progress. The idea that NMDAR signaling is limited to CNS has been challenged in the past two decades. A large body of evidence suggests that NMDAR is expressed in cancer cells outside the CNS and can respond to the autocrine/paracrine release of glutamate. In this review, we survey research on NMDAR signaling and regulation in neurons that can help illuminate its role in tumor biology. Finally, we will discuss existing data on the role of the glutamine/glutamate metabolism, the anticancer action of NMDAR antagonists in experimental models, NMDAR synaptic signaling in tumors, and clinical evidence in human cancer.
Keywords: NMDAR; cancers; glutamate; metabolism; neuron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

