The Significance of Cancer Stem Cells and Epithelial-Mesenchymal Transition in Metastasis and Anti-Cancer Therapy
- PMID: 36768876
- PMCID: PMC9917228
- DOI: 10.3390/ijms24032555
The Significance of Cancer Stem Cells and Epithelial-Mesenchymal Transition in Metastasis and Anti-Cancer Therapy
Abstract
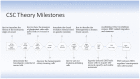
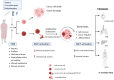
Cancer stem cells (CSCs) have been identified and characterized in both hematopoietic and solid tumors. Their existence was first predicted by Virchow and Cohnheim in the 1870s. Later, many studies showed that CSCs can be identified and isolated by their expression of specific cell markers. The significance of CSCs with respect to tumor biology and anti-cancer treatment lies in their ability to maintain quiescence with very slow proliferation, indefinite self-renewal, differentiation, and trans-differentiation such as epithelial-mesenchymal transition (EMT) and its reverse process mesenchymal-epithelial transition (MET). The ability for detachment, migration, extra- and intravasation, invasion and thereby of completing all necessary steps of the metastatic cascade highlights their significance for metastasis. CSCs comprise the cancer cell populations responsible for tumor growth, resistance to therapies and cancer metastasis. In this review, the history of the CSC theory, their identification and characterization and their biology are described. The contribution of the CSC ability to undergo EMT for cancer metastasis is discussed. Recently, novel strategies for drug development have focused on the elimination of the CSCs specifically. The unique functional and molecular properties of CSCs are discussed as possible therapeutic vulnerabilities for the development of novel anti-metastasis treatments. Prospectively, this may provide precise personalized anti-cancer treatments with improved therapeutic efficiency with fewer side effects and leading to better prognosis.
Keywords: epithelial–mesenchymal transition (EMT); hybrid EMT; invasion; mesenchymal–epithelial transition (MET); partial EMT; stemness; tumor initiating cells; tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Furth J., Kahn M.C., Breedis C. The Transmission of Leukemia of Mice with a Single Cell. Am. J. Cancer. 1937;31:276–282.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

