Influence of PEG Chain Length of Functionalized Magnetic Nanoparticles on the Cytocompatibility and Immune Competence of Primary Murine Macrophages and Dendritic Cells
- PMID: 36768890
- PMCID: PMC9916475
- DOI: 10.3390/ijms24032565
Influence of PEG Chain Length of Functionalized Magnetic Nanoparticles on the Cytocompatibility and Immune Competence of Primary Murine Macrophages and Dendritic Cells
Abstract
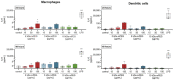
A major drawback of nanoparticles (NPs) for biomedical applications is their preferential phagocytosis in immune cells, which can be avoided by surface modifications like PEGylation. Nevertheless, examinations of different polyethylene glycol (PEG) chain lengths on the competence of immune cells as well as possible immunotoxic effects are still sparse. Therefore, primary murine macrophages and dendritic cells were generated and incubated with magnetic nanoporous silica nanoparticles (MNPSNPs) modified with different mPEG chains (2 kDa, 5 kDa, and 10 kDa). Cytotoxicity, cytokine release, and the formation of reactive oxygen species (ROS) were determined. Immune competence of both cell types was examined and uptake of MNPSNPs into macrophages was visualized. Concentrations up to 150 µg/mL MNPSNPs showed no effects on the metabolic activity or immune competence of both cell types. However, ROS significantly increased in macrophages incubated with larger PEG chains, while the concentration of cytokines (TNF-α and IL-6) did not indicate a proinflammatory process. Investigations on the uptake of MNPSNPs revealed no differences in the onset of internalization and the intensity of intracellular fluorescence. The study gives no indication for an immunotoxic effect of PEGylated MNPSNPs. Nevertheless, there is still a need for optimization regarding their internalization to ensure an efficient drug delivery.
Keywords: Fe3O4; biocompatibility; immunotoxicology; nanoporous silica nanoparticles; phagocytosis; superparamagnetic iron oxide nanoparticles; targeted drug delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Neumann A., Christel A., Kasper C., Behrens P. BMP2-loaded nanoporous silica nanoparticles promote osteogenic differentiation of human mesenchymal stem cells. RSC Adv. 2013;3:24222–24230. doi: 10.1039/c3ra44734k. - DOI
-
- Lin Y.-S., Haynes C.L. Synthesis and Characterization of Biocompatible and Size-Tunable Multifunctional Porous Silica Nanoparticles. Chem. Mater. 2009;21:3979–3986. doi: 10.1021/cm901259n. - DOI
-
- Al-Jamal K.T., Bai J., Wang J.T.-W., Protti A., Southern P., Bogart L., Heidari H., Li X., Cakebread A., Asker D., et al. Magnetic Drug Targeting: Preclinical in Vivo Studies, Mathematical Modeling, and Extrapolation to Humans. Nano Lett. 2016;16:5652–5660. doi: 10.1021/acs.nanolett.6b02261. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

