A Mixture of Chemicals Found in Human Amniotic Fluid Disrupts Brain Gene Expression and Behavior in Xenopus laevis
- PMID: 36768911
- PMCID: PMC9916464
- DOI: 10.3390/ijms24032588
A Mixture of Chemicals Found in Human Amniotic Fluid Disrupts Brain Gene Expression and Behavior in Xenopus laevis
Abstract
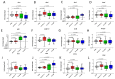
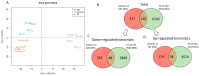
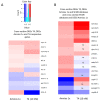
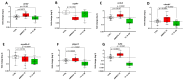
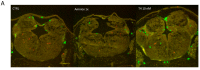
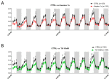
Thyroid hormones (TH) are essential for normal brain development, influencing neural cell differentiation, migration, and synaptogenesis. Multiple endocrine-disrupting chemicals (EDCs) are found in the environment, raising concern for their potential effects on TH signaling and the consequences on neurodevelopment and behavior. While most research on EDCs investigates the effects of individual chemicals, human health may be adversely affected by a mixture of chemicals. The potential consequences of EDC exposure on human health are far-reaching and include problems with immune function, reproductive health, and neurological development. We hypothesized that embryonic exposure to a mixture of chemicals (containing phenols, phthalates, pesticides, heavy metals, and perfluorinated, polychlorinated, and polybrominated compounds) identified as commonly found in the human amniotic fluid could lead to altered brain development. We assessed its effect on TH signaling and neurodevelopment in an amphibian model (Xenopus laevis) highly sensitive to thyroid disruption. Fertilized eggs were exposed for eight days to either TH (thyroxine, T4 10 nM) or the amniotic mixture (at the actual concentration) until reaching stage NF47, where we analyzed gene expression in the brains of exposed tadpoles using both RT-qPCR and RNA sequencing. The results indicate that whilst some overlap on TH-dependent genes exists, T4 and the mixture have different gene signatures. Immunohistochemistry showed increased proliferation in the brains of T4-treated animals, whereas no difference was observed for the amniotic mixture. Further, we demonstrated diminished tadpoles' motility in response to T4 and mixture exposure. As the individual chemicals composing the mixture are considered safe, these results highlight the importance of examining the effects of mixtures to improve risk assessment.
Keywords: Xenopus laevis; endocrine disruption; neurodevelopment; thyroid hormones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

