Clathrin-Mediated Albumin Clearance in Alveolar Epithelial Cells of Murine Precision-Cut Lung Slices
- PMID: 36768968
- PMCID: PMC9916738
- DOI: 10.3390/ijms24032644
Clathrin-Mediated Albumin Clearance in Alveolar Epithelial Cells of Murine Precision-Cut Lung Slices
Abstract
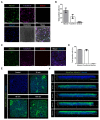
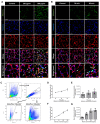
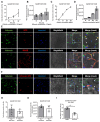
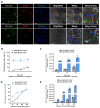
A hallmark of acute respiratory distress syndrome (ARDS) is an accumulation of protein-rich alveolar edema that impairs gas exchange and leads to worse outcomes. Thus, understanding the mechanisms of alveolar albumin clearance is of high clinical relevance. Here, we investigated the mechanisms of the cellular albumin uptake in a three-dimensional culture of precision-cut lung slices (PCLS). We found that up to 60% of PCLS cells incorporated labeled albumin in a time- and concentration-dependent manner, whereas virtually no uptake of labeled dextran was observed. Of note, at a low temperature (4 °C), saturating albumin receptors with unlabeled albumin and an inhibition of clathrin-mediated endocytosis markedly decreased the endocytic uptake of the labeled protein, implicating a receptor-driven internalization process. Importantly, uptake rates of albumin were comparable in alveolar epithelial type I (ATI) and type II (ATII) cells, as assessed in PCLS from a SftpcCreERT2/+: tdTomatoflox/flox mouse strain (defined as EpCAM+CD31-CD45-tdTomatoSPC-T1α+ for ATI and EpCAM+CD31-CD45-tdTomatoSPC+T1α- for ATII cells). Once internalized, albumin was found in the early and recycling endosomes of the alveolar epithelium as well as in endothelial, mesenchymal, and hematopoietic cell populations, which might indicate transcytosis of the protein. In summary, we characterize albumin uptake in alveolar epithelial cells in the complex setting of PCLS. These findings may open new possibilities for pulmonary drug delivery that may improve the outcomes for patients with respiratory failure.
Keywords: acute respiratory distress syndrome; albumin; alveolar epithelium; endocytosis; precision-cut lung slices; protein transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

