A Comprehensive Review on Silk Fibroin as a Persuasive Biomaterial for Bone Tissue Engineering
- PMID: 36768980
- PMCID: PMC9917095
- DOI: 10.3390/ijms24032660
A Comprehensive Review on Silk Fibroin as a Persuasive Biomaterial for Bone Tissue Engineering
Abstract
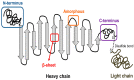
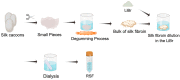
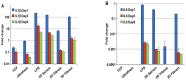
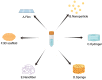
Bone tissue engineering (BTE) utilizes a special mix of scaffolds, cells, and bioactive factors to regulate the microenvironment of bone regeneration and form a three-dimensional bone simulation structure to regenerate bone tissue. Silk fibroin (SF) is perhaps the most encouraging material for BTE given its tunable mechanical properties, controllable biodegradability, and excellent biocompatibility. Numerous studies have confirmed the significance of SF for stimulating bone formation. In this review, we start by introducing the structure and characteristics of SF. After that, the immunological mechanism of SF for osteogenesis is summarized, and various forms of SF biomaterials and the latest development prospects of SF in BTE are emphatically introduced. Biomaterials based on SF have great potential in bone tissue engineering, and this review will serve as a resource for future design and research.
Keywords: biomaterials; bone tissue engineering; silk fibroin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Application of silk fibroin coatings for biomaterial surface modification: a silk road for biomedicine.J Zhejiang Univ Sci B. 2023 Oct 20;24(11):943-956. doi: 10.1631/jzus.B2300003. J Zhejiang Univ Sci B. 2023. PMID: 37961798 Free PMC article. Review.
-
Silk fibroin as biomaterial for bone tissue engineering.Acta Biomater. 2016 Feb;31:1-16. doi: 10.1016/j.actbio.2015.09.005. Epub 2015 Sep 7. Acta Biomater. 2016. PMID: 26360593 Review.
-
Silk fibroin as a potential candidate for bone tissue engineering applications.Biomater Sci. 2025 Jan 14;13(2):364-378. doi: 10.1039/d4bm00950a. Biomater Sci. 2025. PMID: 39620282 Review.
-
Silk Hydrogel for Tissue Engineering: A Review.J Contemp Dent Pract. 2022 Apr 1;23(4):467-477. J Contemp Dent Pract. 2022. PMID: 35945843 Review.
-
Enhanced osteogenesis of β-tricalcium phosphate reinforced silk fibroin scaffold for bone tissue biofabrication.Int J Biol Macromol. 2017 Feb;95:14-23. doi: 10.1016/j.ijbiomac.2016.11.002. Epub 2016 Nov 3. Int J Biol Macromol. 2017. PMID: 27818295
Cited by
-
Investigation of the Nonlinear Optical Properties of Silk Fibroin (SF) Using the Z-Scan Method.Materials (Basel). 2025 Feb 27;18(5):1052. doi: 10.3390/ma18051052. Materials (Basel). 2025. PMID: 40077278 Free PMC article.
-
Electrospun Silk-ICG Composite Fibers and the Application toward Hemorrhage Control.J Funct Biomater. 2024 Sep 19;15(9):272. doi: 10.3390/jfb15090272. J Funct Biomater. 2024. PMID: 39330247 Free PMC article.
-
Protein and Polysaccharide Fibers via Air Jet Spinning: Emerging Techniques for Biomedical and Sustainable Applications.Int J Mol Sci. 2024 Dec 11;25(24):13282. doi: 10.3390/ijms252413282. Int J Mol Sci. 2024. PMID: 39769047 Free PMC article. Review.
-
Evaluation and Application of Silk Fibroin Based Biomaterials to Promote Cartilage Regeneration in Osteoarthritis Therapy.Biomedicines. 2023 Aug 10;11(8):2244. doi: 10.3390/biomedicines11082244. Biomedicines. 2023. PMID: 37626740 Free PMC article. Review.
-
Stimuli-responsive hydrogels for bone tissue engineering.Biomater Transl. 2024 Sep 28;5(3):257-273. doi: 10.12336/biomatertransl.2024.03.004. eCollection 2024. Biomater Transl. 2024. PMID: 39734705 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

