Factors That Control the Force Needed to Unfold a Membrane Protein in Silico Depend on the Mode of Denaturation
- PMID: 36768981
- PMCID: PMC9917119
- DOI: 10.3390/ijms24032654
Factors That Control the Force Needed to Unfold a Membrane Protein in Silico Depend on the Mode of Denaturation
Abstract
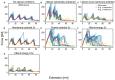
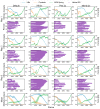
Single-molecule force spectroscopy methods, such as AFM and magnetic tweezers, have proved extremely beneficial in elucidating folding pathways for soluble and membrane proteins. To identify factors that determine the force rupture levels in force-induced membrane protein unfolding, we applied our near-atomic-level Upside molecular dynamics package to study the vertical and lateral pulling of bacteriorhodopsin (bR) and GlpG, respectively. With our algorithm, we were able to selectively alter the magnitudes of individual interaction terms and identify that, for vertical pulling, hydrogen bond strength had the strongest effect, whereas other non-bonded protein and membrane-protein interactions had only moderate influences, except for the extraction of the last helix where the membrane-protein interactions had a stronger influence. The up-down topology of the transmembrane helices caused helices to be pulled out as pairs. The rate-limiting rupture event often was the loss of H-bonds and the ejection of the first helix, which then propagated tension to the second helix, which rapidly exited the bilayer. The pulling of the charged linkers across the membrane had minimal influence, as did changing the bilayer thickness. For the lateral pulling of GlpG, the rate-limiting rupture corresponded to the separation of the helices within the membrane, with the H-bonds generally being broken only afterward. Beyond providing a detailed picture of the rupture events, our study emphasizes that the pulling mode greatly affects the factors that determine the forces needed to unfold a membrane protein.
Keywords: AFM; SMFS; denatured; magnetic tweezers; simulation; unfolded.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

