A Novel Zwitterionic Hydrogel Incorporated with Graphene Oxide for Bone Tissue Engineering: Synthesis, Characterization, and Promotion of Osteogenic Differentiation of Bone Mesenchymal Stem Cells
- PMID: 36769013
- PMCID: PMC9916718
- DOI: 10.3390/ijms24032691
A Novel Zwitterionic Hydrogel Incorporated with Graphene Oxide for Bone Tissue Engineering: Synthesis, Characterization, and Promotion of Osteogenic Differentiation of Bone Mesenchymal Stem Cells
Abstract
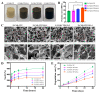
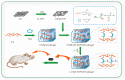
Zwitterionic materials are widely applied in the biomedical field due to their excellent antimicrobial, non-cytotoxicity, and antifouling properties but have never been applied in bone tissue engineering. In this study, we synthesized a novel zwitterionic hydrogel incorporated with graphene oxide (GO) using maleic anhydride (MA) as a cross-linking agent by grafted L-cysteine (L-Cys) as the zwitterionic material on maleilated chitosan via click chemistry. The composition and each reaction procedure of the novel zwitterionic hydrogel were characterized via X-ray diffraction (XRD) and Fourier transformed infrared spectroscopy (FT-IR), while the morphology was imaged by scanning electron microscope (SEM). In vitro cell studies, CCK-8 and live/dead assay, alkaline phosphatase activity, W-B, and qRT-CR tests showed zwitterionic hydrogel incorporated with GO remarkably enhanced the osteogenic differentiation of bone mesenchymal stem cells (BMSCs); it is dose-dependent, and 2 mg/mL GO is the optimum concentration. In vivo tests also indicated the same results. Hence, these results suggested the novel zwitterionic hydrogel exhibited porous characteristics similar to natural bone tissue. In conclusion, the zwitterionic scaffold has highly biocompatible and mechanical properties. When GO was incorporated in this zwitterionic scaffold, the zwitterionic scaffold slows down the release rate and reduces the cytotoxicity of GO. Zwitterions and GO synergistically promote the proliferation and osteogenic differentiation of rBMSCs in vivo and in vitro. The optimal concentration is 2 mg/mL GO.
Keywords: graphene oxide; osteogenic differentiation; stem cell; tissue engineering; zwitterionic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

