Vascular Endothelial Growth Factor as Molecular Target for Bronchopulmonary Dysplasia Prevention in Very Low Birth Weight Infants
- PMID: 36769049
- PMCID: PMC9916882
- DOI: 10.3390/ijms24032729
Vascular Endothelial Growth Factor as Molecular Target for Bronchopulmonary Dysplasia Prevention in Very Low Birth Weight Infants
Abstract
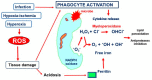
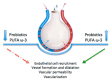
Bronchopulmonary dysplasia (BPD) still represents an important burden of neonatal care. The definition of the disease is currently undergoing several revisions, and, to date, BPD is actually defined by its treatment rather than diagnostic or clinic criteria. BPD is associated with many prenatal and postnatal risk factors, such as maternal smoking, chorioamnionitis, intrauterine growth restriction (IUGR), patent ductus arteriosus (PDA), parenteral nutrition, sepsis, and mechanical ventilation. Various experimental models have shown how these factors cause distorted alveolar and vascular growth, as well as alterations in the composition and differentiation of the mesenchymal cells of a newborn's lungs, demonstrating a multifactorial pathogenesis of the disease. In addition, inflammation and oxidative stress are the common denominators of the mechanisms that contribute to BPD development. Vascular endothelial growth factor-A (VEGFA) constitutes the most prominent and best studied candidate for vascular development. Animal models have confirmed the important regulatory roles of epithelial-expressed VEGF in lung development and function. This educational review aims to discuss the inflammatory pathways in BPD onset for preterm newborns, focusing on the role of VEGFA and providing a summary of current and emerging evidence.
Keywords: BPD; VEGFA; biomarker; inflammation; lung disease; oxidative stress; preterm.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Holzfurtner L., Shahzad T., Dong Y., Rekers L., Selting A., Staude B., Lauer T., Schmidt A., Rivetti S., Zimmer K.-P., et al. When Inflammation Meets Lung Development-an Update on the Pathogenesis of Bronchopulmonary Dysplasia. Mol. Cell Pediatr. 2022;9:7. doi: 10.1186/s40348-022-00137-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

