Significance of Singlet Oxygen Molecule in Pathologies
- PMID: 36769060
- PMCID: PMC9917472
- DOI: 10.3390/ijms24032739
Significance of Singlet Oxygen Molecule in Pathologies
Abstract
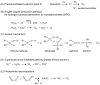
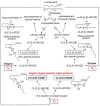
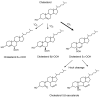
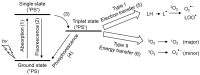
Reactive oxygen species, including singlet oxygen, play an important role in the onset and progression of disease, as well as in aging. Singlet oxygen can be formed non-enzymatically by chemical, photochemical, and electron transfer reactions, or as a byproduct of endogenous enzymatic reactions in phagocytosis during inflammation. The imbalance of antioxidant enzymes and antioxidant networks with the generation of singlet oxygen increases oxidative stress, resulting in the undesirable oxidation and modification of biomolecules, such as proteins, DNA, and lipids. This review describes the molecular mechanisms of singlet oxygen production in vivo and methods for the evaluation of damage induced by singlet oxygen. The involvement of singlet oxygen in the pathogenesis of skin and eye diseases is also discussed from the biomolecular perspective. We also present our findings on lipid oxidation products derived from singlet oxygen-mediated oxidation in glaucoma, early diabetes patients, and a mouse model of bronchial asthma. Even in these diseases, oxidation products due to singlet oxygen have not been measured clinically. This review discusses their potential as biomarkers for diagnosis. Recent developments in singlet oxygen scavengers such as carotenoids, which can be utilized to prevent the onset and progression of disease, are also described.
Keywords: biomarkers; lipid peroxidation; reactive oxygen species; singlet oxygen.
Conflict of interest statement
Yasukazu Yoshida is an employee of LG Japan Lab, Inc. This paper has not received any form of financial support from LG Japan Lab, Inc. The findings of this paper do not result in any commercial or economic interest to LG Japan Lab, Inc. or any of the authors.
Figures





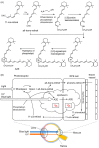
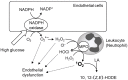

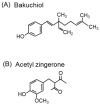
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

