Stimuli-Responsive Boron-Based Materials in Drug Delivery
- PMID: 36769081
- PMCID: PMC9917063
- DOI: 10.3390/ijms24032757
Stimuli-Responsive Boron-Based Materials in Drug Delivery
Abstract
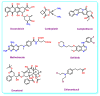
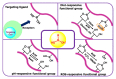
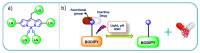
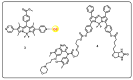
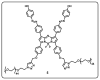
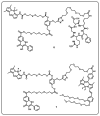
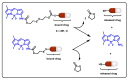
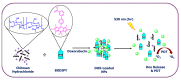
Drug delivery systems, which use components at the nanoscale level as diagnostic tools or to release therapeutic drugs to particular target areas in a regulated manner, are a fast-evolving field of science. The active pharmaceutical substance can be released via the drug delivery system to produce the desired therapeutic effect. The poor bioavailability and irregular plasma drug levels of conventional drug delivery systems (tablets, capsules, syrups, etc.) prevent them from achieving sustained delivery. The entire therapy process may be ineffective without a reliable delivery system. To achieve optimal safety and effectiveness, the drug must also be administered at a precision-controlled rate and the targeted spot. The issues with traditional drug delivery are overcome by the development of stimuli-responsive controlled drug release. Over the past decades, regulated drug delivery has evolved considerably, progressing from large- and nanoscale to smart-controlled drug delivery for several diseases. The current review provides an updated overview of recent developments in the field of stimuli-responsive boron-based materials in drug delivery for various diseases. Boron-containing compounds such as boron nitride, boronic acid, and boron dipyrromethene have been developed as a moving field of research in drug delivery. Due to their ability to achieve precise control over drug release through the response to particular stimuli (pH, light, glutathione, glucose or temperature), stimuli-responsive nanoscale drug delivery systems are attracting a lot of attention. The potential of developing their capabilities to a wide range of nanoscale systems, such as nanoparticles, nanosheets/nanospheres, nanotubes, nanocarriers, microneedles, nanocapsules, hydrogel, nanoassembly, etc., is also addressed and examined. This review also provides overall design principles to include stimuli-responsive boron nanomaterial-based drug delivery systems, which might inspire new concepts and applications.
Keywords: BODIPY; boron; boron nitrite; boronic acid; drug delivery; stimuli-responsive.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

