Synthesis, Biological and In Silico Studies of Griseofulvin and Usnic Acid Sulfonamide Derivatives as Fungal, Bacterial and Human Carbonic Anhydrase Inhibitors
- PMID: 36769114
- PMCID: PMC9917406
- DOI: 10.3390/ijms24032802
Synthesis, Biological and In Silico Studies of Griseofulvin and Usnic Acid Sulfonamide Derivatives as Fungal, Bacterial and Human Carbonic Anhydrase Inhibitors
Abstract
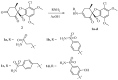
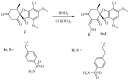
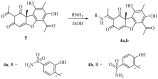
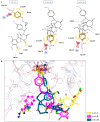
Carbonic anhydrases (CAs, EC 4.2.1.1) catalyze the essential reaction of CO2 hydration in all living organisms, being actively involved in the regulation of a plethora of patho-/physiological conditions. A series of griseofulvin and usnic acid sulfonamides were synthesized and tested as possible CA inhibitors. Since β- and γ- classes are expressed in microorganisms in addition to the α- class, showing substantial structural differences to the human isoforms they are also interesting as new antiinfective targets with a different mechanism of action for fighting the emerging problem of extensive drug resistance afflicting most countries worldwide. Griseofulvin and usnic acid sulfonamides were synthesized using methods of organic chemistry. Their inhibitory activity, assessed against the cytosolic human isoforms hCA I and hCA II, the transmembrane hCA IX as well as β- and γ-CAs from different bacterial and fungal strains, was evaluated by a stopped-flow CO2 hydrase assay. Several of the investigated derivatives showed interesting inhibition activity towards the cytosolic associate isoforms hCA I and hCA II, as well as the three γ-CAs and Malassezia globosa (MgCA) enzyme. Six compounds (1b-1d, 1h, 1i and 1j) were more potent than AAZ against hCA I while five (1d, 1h, 1i, 1j and 4a) showed better activity than AAZ against the hCA II isoform. Moreover, all compounds appeared to be very potent against MgCA with a Ki lower than that of the reference drug. Furthermore, computational procedures were used to investigate the binding mode of this class of compounds within the active site of human CAs.
Keywords: carbonic anhydrase inhibitors; griseofulvin derivatives; in silico studies; stopped-flow CO2 hydrase assay; usnic acid derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Supuran C.T., McKenna R. Carbonic anhydrase inhibitors drug design. In: McKenna R., Frost S., editors. Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications. Springer; Berlin/Heidelberg, Germany: 2014. pp. 291–323. - PubMed
-
- Pacchiano F., Carta F., McDonald P.C., Lou Y., Vullo D., Scozzafava A., Dedhar S., Supuran C.T. Ureido-substituted benzenesulfonamides potently inhibit carbonic anhydrase IX and show antimetastatic activity in a model of breast cancer metastasis. J. Med. Chem. 2011;54:1896–1902. doi: 10.1021/jm101541x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

