Characterization of Hormone Receptor and HER2 Status in Breast Cancer Using Mass Spectrometry Imaging
- PMID: 36769215
- PMCID: PMC9918176
- DOI: 10.3390/ijms24032860
Characterization of Hormone Receptor and HER2 Status in Breast Cancer Using Mass Spectrometry Imaging
Abstract
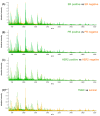
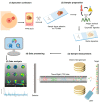
Immunohistochemical evaluation of estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 status stratify the different subtypes of breast cancer and define the treatment course. Triple-negative breast cancer (TNBC), which does not register receptor overexpression, is often associated with worse patient prognosis. Mass spectrometry imaging transcribes the molecular content of tissue specimens without requiring additional tags or preliminary analysis of the samples, being therefore an excellent methodology for an unbiased determination of tissue constituents, in particular tumor markers. In this study, the proteomic content of 1191 human breast cancer samples was characterized by mass spectrometry imaging and the epithelial regions were employed to train and test machine-learning models to characterize the individual receptor status and to classify TNBC. The classification models presented yielded high accuracies for estrogen and progesterone receptors and over 95% accuracy for classification of TNBC. Analysis of the molecular features revealed that vimentin overexpression is associated with TNBC, supported by immunohistochemistry validation, revealing a new potential target for diagnosis and treatment.
Keywords: breast cancer; histopathology; mass spectrometry imaging; proteomics; tissue typing.
Conflict of interest statement
The RapifleX MALDI Tissuetyper TOF mass spectrometer was provided by Bruker Daltonics GmbH as part of a collaboration agreement between Bruker Daltonics GmbH and the Technical University of Munich. P.J. reports stock ownership in Myriad Genetics, Inc. S.L. has received funding from Abbvie, Amgen, Astra Zeneca, Celgene, Novartis, Pfizer, Roche, Seattle Genetics, PriME/Medscape, Teva, Daiichi-Sankyo, Vifor, Samsung, Lilly, Eirgenix, BMS, Puma, MSD, and Immunomedics, and personal fees from Chugai; in addition, S.L. has a patent EP14153692.0 pending. V.N. declares to be GBG Forschungs GmbH employee. GBG Forschungs GmbH received funding for research grants from Abbvie, AstraZeneca, BMS, Daiichi-Sankyo, Gilead, Novartis, Pfizer and Roche (paid to the institution); other (non-financial/medical writing) assistance was received from Daiichi-Sankyo, Gilead, Novartis, Pfizer, Roche and Seagen (paid to the institution). GBG Forschungs GmbH has the following royalties/patents: EP14153692.0, EP21152186.9, EP15702464.7, EP19808852.8 and VM Scope GmbH; P.F. reports personal fees from Novartis, Daiichi-Sankyo, Astra Zeneca, Eisai, Merck Sharp & Dohme, Lilly, Pierre Fabre, SeaGen, Roche, Agendia, Sanofi Aventis, and Gilead, grants from BioNtech, and Cepheid, and grants and personal fees from Pfizer. F.M. has attended Advisory Boards and/or served as speaker for AstraZeneca, Clovis, Daiichi Sankyo, EISAI, GenomicHealth, Gilead/immunomedics, GSK, GSK/Tesaro, Immunicom, Lilly, MSD, Myriad, Novartis, Pfizer, PharmaMar, Roche, and Seagen. F.M. also declares institutional activities without financial interest in AGO Research GmbH, AstraZeneca, Aisai, German Breast Group, Gilead/Immunomedics, GSK, Lilly, MSD, Novartis, Roche, Seagen, and Vaccibody. C.D. reports grants from European Commission H2020, German Cancer Aid Translational Oncology, German Breast Group; personal fees from Novartis, Roche, MSD Oncology, Daiichi Sankyo, AstraZeneca, Molecular Health, Merck, grants from Myriad to his institution, other from Sividon diagnostics (cofounder and former shareholder until 2016); in addition, C.D. has a patent VMScope digital pathology software with royalties paid, a patent WO2020109570A1 (cancer immunotherapy; pending), and a patent WO2015114146A1 and WO2010076322A1 (therapy response issued). W.W. has attended Advisory Boards and served as speaker for Roche, MSD, BMS, AstraZeneca, Pfizer, Merck, Lilly, Boehringer, Novartis, Takeda, Bayer, Amgen, Astellas, Eisai, Illumina, Siemens, Agilent, ADC, GSK and Molecular Health. W.W. receives research funding from Roche, MSD, BMS and AstraZeneca. K.S. has attended Advisory Boards and served as speaker for Roche, BMS, MSD and Merck. All other authors declare that no conflicts of interests exist.
Figures


Similar articles
-
Distribution of molecular breast cancer subtypes among Algerian women and correlation with clinical and tumor characteristics: a population-based study.Breast Dis. 2015;35(2):95-102. doi: 10.3233/BD-150398. Breast Dis. 2015. PMID: 25736840
-
Expression of SIRT1, SIRT3 and SIRT6 Genes for Predicting Survival in Triple-Negative and Hormone Receptor-Positive Subtypes of Breast Cancer.Pathol Oncol Res. 2020 Oct;26(4):2723-2731. doi: 10.1007/s12253-020-00873-5. Epub 2020 Jul 17. Pathol Oncol Res. 2020. PMID: 32681437
-
The Prognosis and Predictive Value of Estrogen Negative/Progesterone Positive (ER-/PR+) Phenotype: Experience of 1159 Primary Breast Cancer from a Single Institute.Breast J. 2022 May 17;2022:9238804. doi: 10.1155/2022/9238804. eCollection 2022. Breast J. 2022. PMID: 35711896 Free PMC article.
-
'Triple negative' epithelial ovarian cancer and pathologic markers for prognosis.Curr Opin Obstet Gynecol. 2011 Feb;23(1):19-23. doi: 10.1097/GCO.0b013e32834252f5. Curr Opin Obstet Gynecol. 2011. PMID: 21150601 Review.
-
Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: Single-institution experience and review of published literature.Eur J Surg Oncol. 2017 Apr;43(4):642-648. doi: 10.1016/j.ejso.2016.10.025. Epub 2016 Nov 17. Eur J Surg Oncol. 2017. PMID: 27889196 Review.
Cited by
-
Deep learning application in prediction of cancer molecular alterations based on pathological images: a bibliographic analysis via CiteSpace.J Cancer Res Clin Oncol. 2024 Oct 18;150(10):467. doi: 10.1007/s00432-024-05992-z. J Cancer Res Clin Oncol. 2024. PMID: 39422817 Free PMC article.
-
Unraveling Biomarker Signatures in Triple-Negative Breast Cancer: A Systematic Review for Targeted Approaches.Int J Mol Sci. 2024 Feb 22;25(5):2559. doi: 10.3390/ijms25052559. Int J Mol Sci. 2024. PMID: 38473804 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

