Protein Susceptibility to Peroxidation by 4-Hydroxynonenal in Hereditary Hemochromatosis
- PMID: 36769239
- PMCID: PMC9917916
- DOI: 10.3390/ijms24032922
Protein Susceptibility to Peroxidation by 4-Hydroxynonenal in Hereditary Hemochromatosis
Abstract
Iron overload caused by hereditary hemochromatosis (HH) increases free reactive oxygen species that, in turn, induce lipid peroxidation. Its 4-hydroxynonenal (HNE) by-product is a well-established marker of lipid peroxidation since it reacts with accessible proteins with deleterious consequences. Indeed, elevated levels of HNE are often detected in a wide variety of human diseases related to oxidative stress. Here, we evaluated HNE-modified proteins in the membrane of erythrocytes from HH patients and in organs of Hfe-/- male and female mice, a mouse model of HH. For this purpose, we used one- and two-dimensional gel electrophoresis, immunoblotting and MALDI-TOF/TOF analysis. We identified cytoskeletal membrane proteins and membrane receptors of erythrocytes bound to HNE exclusively in HH patients. Furthermore, kidney and brain of Hfe-/- mice contained more HNE-adducted protein than healthy controls. Our results identified main HNE-modified proteins suggesting that HH favours preferred protein targets for oxidation by HNE.
Keywords: 4-hydroxynonenal (HNE); Hfe−/− mouse; erythrocyte membrane proteins; hemochromatosis; lipid peroxidation; oxidative stress; protein modification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

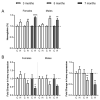

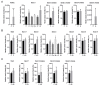
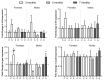
References
-
- Papanikolaou G., Samuels M.E., Ludwig E.H., MacDonald M.L.E., Franchini P.L., Dubé M.-P., Andres L., MacFarlane J., Sakellaropoulos N., Politou M., et al. Mutations in HFE2 cause iron overload in chromosome 1q–linked juvenile hemochromatosis. Nat. Genet. 2004;36:77–82. doi: 10.1038/ng1274. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

