ER-PM Junctions on GABAergic Interneurons Are Organized by Neuregulin 2/VAP Interactions and Regulated by NMDA Receptors
- PMID: 36769244
- PMCID: PMC9917868
- DOI: 10.3390/ijms24032908
ER-PM Junctions on GABAergic Interneurons Are Organized by Neuregulin 2/VAP Interactions and Regulated by NMDA Receptors
Abstract
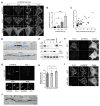
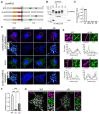
Neuregulins (NRGs) signal via ErbB receptors to regulate neural development, excitability, synaptic and network activity, and behaviors relevant to psychiatric disorders. Bidirectional signaling between NRG2/ErbB4 and NMDA receptors is thought to homeostatically regulate GABAergic interneurons in response to increased excitatory neurotransmission or elevated extracellular glutamate levels. Unprocessed proNRG2 forms discrete clusters on cell bodies and proximal dendrites that colocalize with the potassium channel Kv2.1 at specialized endoplasmic reticulum-plasma membrane (ER-PM) junctions, and NMDA receptor activation triggers rapid dissociation from ER-PM junctions and ectodomain shedding by ADAM10. Here, we elucidate the mechanistic basis of proNRG2 clustering at ER-PM junctions and its regulation by NMDA receptors. Importantly, we demonstrate that proNRG2 promotes the formation of ER-PM junctions by directly binding the ER-resident membrane tether VAP, like Kv2.1. The proNRG2 intracellular domain harbors two non-canonical, low-affinity sites that cooperatively mediate VAP binding. One of these is a cryptic and phosphorylation-dependent VAP binding motif that is dephosphorylated following NMDA receptor activation, thus revealing how excitatory neurotransmission promotes the dissociation of proNRG2 from ER-PM junctions. Therefore, proNRG2 and Kv2.1 can independently function as VAP-dependent organizers of neuronal ER-PM junctions. Based on these and prior studies, we propose that proNRG2 and Kv2.1 serve as co-regulated downstream effectors of NMDA receptors to homeostatically regulate GABAergic interneurons.
Keywords: ER-PM junction; FFAT; GABAergic interneuron; Kv2.1; NMDA receptor; VAP; neuregulin 2.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

