Advanced Glycation End-Products and Diabetic Neuropathy of the Retina
- PMID: 36769249
- PMCID: PMC9917392
- DOI: 10.3390/ijms24032927
Advanced Glycation End-Products and Diabetic Neuropathy of the Retina
Abstract
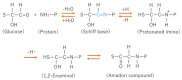
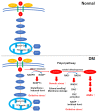
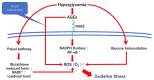
Diabetic retinopathy is a tissue-specific neurovascular impairment of the retina in patients with both type 1 and type 2 diabetes. Several pathological factors are involved in the progressive impairment of the interdependence between cells that consist of the neurovascular units (NVUs). The advanced glycation end-products (AGEs) are one of the major pathological factors that cause the impairments of neurovascular coupling in diabetic retinopathy. Although the exact mechanisms for the toxicities of the AGEs in diabetic retinopathy have not been definitively determined, the AGE-receptor of the AGE (RAGE) axis, production of reactive oxygen species, inflammatory reactions, and the activation of the cell death pathways are associated with the impairment of the NVUs in diabetic retinopathy. More specifically, neuronal cell death is an irreversible change that is directly associated with vision reduction in diabetic patients. Thus, neuroprotective therapies must be established for diabetic retinopathy. The AGEs are one of the therapeutic targets to examine to ameliorate the pathological changes in the NVUs in diabetic retinopathy. This review focuses on the basic and pathological findings of AGE-induced neurovascular abnormalities and the potential therapeutic approaches, including the use of anti-glycated drugs to protect the AGE-induced impairments of the NVUs in diabetic retinopathy.
Keywords: advanced glycation end-products; anti-glycation; apoptosis; diabetic retinopathy; neuroprotection; neurovascular unit; nutrients; oxidative stress; polyol pathway; receptor of AGEs.
Conflict of interest statement
The author declares no conflict of interest.
Figures
References
-
- Leasher J.L., Bourne R.R., Flaxman S.R., Jonas J.B., Keeffe J., Naidoo K., Pesudovs K., Price H., White R.A., Wong T.Y., et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: A meta-analysis from 1990 to 2010. Diabetes Care. 2016;39:1643–1649. doi: 10.2337/dc15-2171. - DOI - PubMed
-
- Yau J.W., Rogers S.L., Kawasaki R., Lamoureux E.L., Kowalski J.W., Bek T., Chen S.J., Dekker J.M., Fletcher A., Grauslund J., et al. Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

