Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications
- PMID: 36769260
- PMCID: PMC9917539
- DOI: 10.3390/ijms24032937
Piperidine Derivatives: Recent Advances in Synthesis and Pharmacological Applications
Abstract
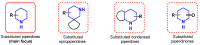
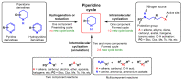
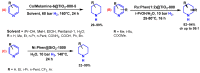
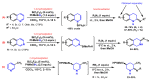
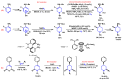
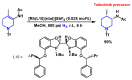
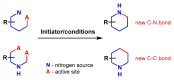
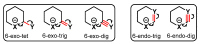
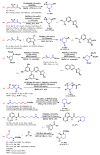
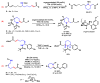
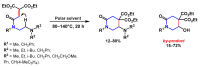
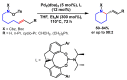
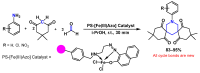
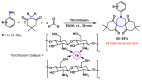
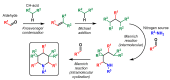
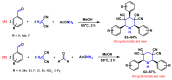
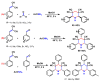
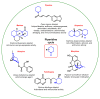
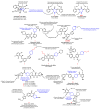
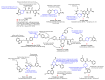
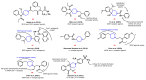
Piperidines are among the most important synthetic fragments for designing drugs and play a significant role in the pharmaceutical industry. Their derivatives are present in more than twenty classes of pharmaceuticals, as well as alkaloids. The current review summarizes recent scientific literature on intra- and intermolecular reactions leading to the formation of various piperidine derivatives: substituted piperidines, spiropiperidines, condensed piperidines, and piperidinones. Moreover, the pharmaceutical applications of synthetic and natural piperidines were covered, as well as the latest scientific advances in the discovery and biological evaluation of potential drugs containing piperidine moiety. This review is designed to help both novice researchers taking their first steps in this field and experienced scientists looking for suitable substrates for the synthesis of biologically active piperidines.
Keywords: amination; annulation; biological activity; cyclization; cycloaddition; hydrogenation; multicomponent reactions; pharmacological activity; piperidines; piperidinones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
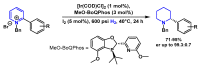


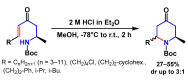
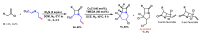
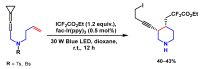

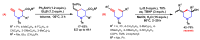
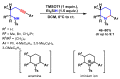

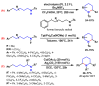
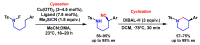
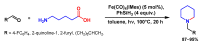

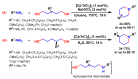
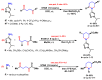
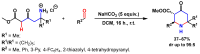



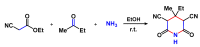

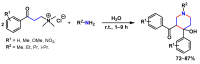

References
-
- Sandmeier T., Carreira E.M. Synthetic Approaches to Nonaromatic Nitrogen Heterocycles. John Wiley & Sons Ltd.; Hoboken, NJ, USA: 2020. Modern Catalytic Enantioselective Approaches to Piperidines; pp. 249–271. Chapter 10. - DOI
-
- Díez-Poza C., Barbero H., Diez-Varga A., Barbero A. Chapter 2–The Silyl-Prins Reaction as an Emerging Method for the Synthesis of Heterocycles. In: Gribble G.W., Joule J.A., editors. Progress in Heterocyclic Chemistry. Volume 30. Elsevier; Amsterdam, The Netherlands: 2018. pp. 13–41.
-
- Kaur G., Devi M., Kumari A., Devi R., Banerjee B. One-Pot Pseudo Five Component Synthesis of Biologically Relevant 1,2,6-Triaryl-4-arylamino-piperidine-3-ene-3-carboxylates: A Decade Update. ChemistrySelect. 2018;3:9892–9910. doi: 10.1002/slct.201801887. - DOI
-
- Kaur G., Devi P., Thakur S., Kumar A., Chandel R., Banerjee B. Magnetically Separable Transition Metal Ferrites: Versatile Heterogeneous Nano-Catalysts for the Synthesis of Diverse Bioactive Heterocycles. ChemistrySelect. 2019;4:2181–2199. doi: 10.1002/slct.201803600. - DOI
-
- Park S. B(C6F5)3-Catalyzed sp3 C—Si Bond Forming Consecutive Reactions. Chin. J. Chem. 2019;37:1057–1071. doi: 10.1002/cjoc.201900240. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

