The In Vitro Impact of Isoxazole Derivatives on Pathogenic Biofilm and Cytotoxicity of Fibroblast Cell Line
- PMID: 36769319
- PMCID: PMC9917413
- DOI: 10.3390/ijms24032997
The In Vitro Impact of Isoxazole Derivatives on Pathogenic Biofilm and Cytotoxicity of Fibroblast Cell Line
Abstract
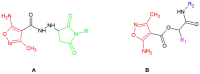
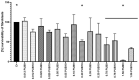
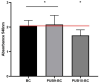
The microbial, biofilm-based infections of chronic wounds are one of the major challenges of contemporary medicine. The use of topically administered antiseptic agents is essential to treat wound-infecting microorganisms. Due to observed microbial tolerance/resistance against specific clinically-used antiseptics, the search for new, efficient agents is of pivotal meaning. Therefore, in this work, 15 isoxazole derivatives were scrutinized against leading biofilm wound pathogens Staphylococcus aureus and Pseudomonas aeruginosa, and against Candida albicans fungus. For this purpose, the minimal inhibitory concentration, biofilm reduction in microtitrate plates, modified disk diffusion methods and antibiofilm dressing activity measurement methods were applied. Moreover, the cytotoxicity and cytocompatibility of derivatives was tested toward wound bed-forming cells, referred to as fibroblasts, using normative methods. Obtained results revealed that all isoxazole derivatives displayed antimicrobial activity and low cytotoxic effect, but antimicrobial activity of two derivatives, 2-(cyclohexylamino)-1-(5-nitrothiophen-2-yl)-2-oxoethyl 5-amino-3-methyl-1,2-oxazole-4-carboxylate (PUB9) and 2-(benzylamino)-1-(5-nitrothiophen-2-yl)-2-oxoethyl 5-amino-3-methyl-1,2-oxazole-4-carboxylate (PUB10), was noticeably higher compared to the other compounds analyzed, especially PUB9 with regard to Staphylococcus aureus, with a minimal inhibitory concentration more than x1000 lower compared to the remaining derivatives. The PUB9 and PUB10 derivatives were able to reduce more than 90% of biofilm-forming cells, regardless of the species, displaying at the same time none (PUB9) or moderate (PUB10) cytotoxicity against fibroblasts and high (PUB9) or moderate (PUB10) cytocompatibility against these wound cells. Therefore, taking into consideration the clinical demand for new antiseptic agents for non-healing wound treatment, PUB9 seems to be a promising candidate to be further tested in advanced animal models and later, if satisfactory results are obtained, in the clinical setting.
Keywords: Michael addition; Passerini reaction; anti-bacterial activity; antiseptics; cytotoxicity; isoxazole.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
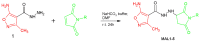


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

