The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment
- PMID: 36769332
- PMCID: PMC9917762
- DOI: 10.3390/ijms24033015
The EPH/Ephrin System in Pancreatic Ductal Adenocarcinoma (PDAC): From Pathogenesis to Treatment
Abstract
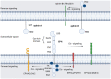
Pancreatic ductal adenocarcinoma (PDAC) is a major concern for health care systems worldwide, since its mortality remains unaltered despite the surge in cutting-edge science. The EPH/ephrin signaling system was first investigated in the 1980s. EPH/ephrins have been shown to exert bidirectional signaling and cell-to-cell communication, influencing cellular morphology, adhesion, migration and invasion. Recent studies have highlighted the critical role of the EPH/ephrin system in various physiologic processes, including cellular proliferation, survival, synaptic plasticity and angiogenesis. Thus, it has become evident that the EPH/ephrin signaling system may have compelling effects on cell homeostasis that contribute to carcinogenesis. In particular, the EPH/ephrins have an impact on pancreatic morphogenesis and development, whereas several EPHs and ephrins are altered in PDAC. Several clinical and preclinical studies have attempted to elucidate the effects of the EPH/ephrin pathway, with multilayered effects on PDAC development. These studies have highlighted its highly promising role in the diagnosis, prognosis and therapeutic management of PDAC. The aim of this review is to explore the obscure aspects of the EPH/ephrin system concerning the development, physiology and homeostasis of the pancreas.
Keywords: EPH/eprin; PDAC; immunotherapy; pancreatic cancer; signaling pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Wang S., Zheng Y., Yang F., Zhu L., Zhu X.Q., Wang Z.F., Wu X.L., Zhou C.H., Yan J.Y., Hu B.Y., et al. The molecular biology of pancreatic adenocarcinoma: Translational challenges and clinical perspectives. Signal Transduct. Target. Ther. 2021;6:249. doi: 10.1038/s41392-021-00659-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

