GLI1 Deficiency Impairs the Tendon-Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice
- PMID: 36769647
- PMCID: PMC9917856
- DOI: 10.3390/jcm12030999
GLI1 Deficiency Impairs the Tendon-Bone Healing after Anterior Cruciate Ligament Reconstruction: In Vivo Study Using Gli1-Transgenic Mice
Abstract
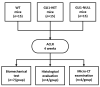
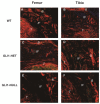
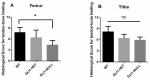
Hedgehog (Hh) signaling plays a fundamental role in the enthesis formation process and GLI-Kruppel family member GLI1 (Gli1) is a key downstream mediator. However, the role of Gli1 in tendon-bone healing after anterior cruciate ligament reconstruction (ACLR) is unknown. To evaluate the tendon-bone healing after ACLR in Gli1LacZ/LacZ (GLI1-NULL) mice, and compare Gli1LacZ/WT (GLI1-HET) and Gli1WT/WT wild type (WT) mice, a total of 45 mice, 15 mice each of GLI1-NULL, GLI1-HET and WT were used in this study. All mice underwent microsurgical ACLR at 12 weeks of age. Mice were euthanized at 4 weeks after surgery and were used for biomechanical testing, histological evaluation, and micro-CT analysis. The GLI1-NULL group had significantly lower biomechanical failure force, poorer histological healing, and lower BV/TV when compared with the WT and GLI1-HET groups. These significant differences were only observed at the femoral tunnel. Immunohistology staining showed positive expression of Indian hedgehog (IHH) and Patched 1(PTCH1) in all three groups, which indicated the activation of the Hh signal pathway. The GLI1 was negative in the GLI1-NULL group, validating the absence of GLI1 protein in these mice. These results proved that activation of the Hh signaling pathway occurs during ACL graft healing, and the function of Gli1 was necessary for tendon-bone healing. Healing in the femoral tunnel is more obviously impaired by Gli1 deficiency. Our findings provide further insight into the molecular mechanism of tendon-bone healing and suggest that Gli1 might represent a novel therapeutic target to improve tendon-bone healing after ACLR.
Keywords: Gli1; anterior cruciate ligament reconstruction; hedgehog; tendon–bone healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Webster K.E., Feller J.A., Hameister K.A. Bone tunnel enlargement following anterior cruciate ligament reconstruction: A randomised comparison of hamstring and patellar tendon grafts with 2-year follow-up. Knee Surg. Sports Traumatol. Arthrosc. 2001;9:86–91. doi: 10.1007/s001670100191. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

