Multi-Parametric Exploration of a Selection of Piezoceramic Materials for Bone Graft Substitute Applications
- PMID: 36769908
- PMCID: PMC9917895
- DOI: 10.3390/ma16030901
Multi-Parametric Exploration of a Selection of Piezoceramic Materials for Bone Graft Substitute Applications
Abstract
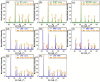
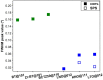
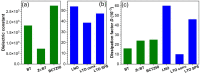
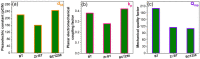
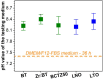
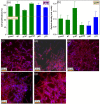
This work was devoted to the first multi-parametric unitary comparative analysis of a selection of sintered piezoceramic materials synthesised by solid-state reactions, aiming to delineate the most promising biocompatible piezoelectric material, to be further implemented into macro-porous ceramic scaffolds fabricated by 3D printing technologies. The piezoceramics under scrutiny were: KNbO3, LiNbO3, LiTaO3, BaTiO3, Zr-doped BaTiO3, and the (Ba0.85Ca0.15)(Ti0.9Zr0.1)O3 solid solution (BCTZ). The XRD analysis revealed the high crystallinity of all sintered ceramics, while the best densification was achieved for the BaTiO3-based materials via conventional sintering. Conjunctively, BCTZ yielded the best combination of functional properties-piezoelectric response (in terms of longitudinal piezoelectric constant and planar electromechanical coupling factor) and mechanical and in vitro osteoblast cell compatibility. The selected piezoceramic was further used as a base material for the robocasting fabrication of 3D macro-porous scaffolds (porosity of ~50%), which yielded a promising compressive strength of ~20 MPa (higher than that of trabecular bone), excellent cell colonization capability, and noteworthy cytocompatibility in osteoblast cell cultures, analogous to the biological control. Thereby, good prospects for the possible development of a new generation of synthetic bone graft substitutes endowed with the piezoelectric effect as a stimulus for the enhancement of osteogenic capacity were settled.
Keywords: bone graft substitutes; in vitro testing; macro-porous scaffolds; physico-chemical characterization; piezoceramics; robocasting.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Optimization of Processing Steps for Superior Functional Properties of (Ba, Ca)(Zr, Ti)O3 Ceramics.Materials (Basel). 2022 Dec 9;15(24):8809. doi: 10.3390/ma15248809. Materials (Basel). 2022. PMID: 36556617 Free PMC article.
-
Fabrication and characterization of highly porous barium titanate based scaffold coated by Gel/HA nanocomposite with high piezoelectric coefficient for bone tissue engineering applications.J Mech Behav Biomed Mater. 2018 Mar;79:195-202. doi: 10.1016/j.jmbbm.2017.12.034. Epub 2017 Dec 30. J Mech Behav Biomed Mater. 2018. PMID: 29306083
-
Boosting Upconversion Photoluminescence and Multielectrical Properties via Er-Doping-Modulated Vacancy Control in Ba0.85Ca0.15Ti0.9Zr0.1O3.ACS Omega. 2019 Jun 24;4(6):11004-11013. doi: 10.1021/acsomega.9b01391. eCollection 2019 Jun 30. ACS Omega. 2019. PMID: 31460198 Free PMC article.
-
3D printed porous ceramic scaffolds for bone tissue engineering: a review.Biomater Sci. 2017 Aug 22;5(9):1690-1698. doi: 10.1039/c7bm00315c. Biomater Sci. 2017. PMID: 28686244 Review.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
Cited by
-
Advanced Piezoelectric Materials, Devices, and Systems for Orthopedic Medicine.Adv Sci (Weinh). 2025 Jan;12(3):e2410400. doi: 10.1002/advs.202410400. Epub 2024 Dec 12. Adv Sci (Weinh). 2025. PMID: 39665130 Free PMC article. Review.
-
Insights into Antisite Defect Complex Induced High Ferro-Piezoelectric Properties in KNbO3 Perovskite: First-Principles Study.Materials (Basel). 2024 Jul 11;17(14):3442. doi: 10.3390/ma17143442. Materials (Basel). 2024. PMID: 39063734 Free PMC article.
-
Piezoelectric biomaterials for providing electrical stimulation in bone tissue engineering: Barium titanate.J Orthop Translat. 2025 Feb 4;51:94-107. doi: 10.1016/j.jot.2024.12.011. eCollection 2025 Mar. J Orthop Translat. 2025. PMID: 39991455 Free PMC article. Review.
-
Ginger extract loaded Fe2O3/MgO-doped hydroxyapatite: Evaluation of biological properties for bone-tissue engineering.J Am Ceram Soc. 2024 Apr;107(4):2081-2092. doi: 10.1111/jace.19568. Epub 2023 Dec 8. J Am Ceram Soc. 2024. PMID: 38855017 Free PMC article.
-
Two decades of continuous progresses and breakthroughs in the field of bioactive ceramics and glasses driven by CICECO-hub scientists.Bioact Mater. 2024 Jun 8;40:104-147. doi: 10.1016/j.bioactmat.2024.05.041. eCollection 2024 Oct. Bioact Mater. 2024. PMID: 39659434 Free PMC article. Review.
References
-
- World Health Organization Life Expencancy and Healthy Life Expectancy. [(accessed on 28 November 2022)]. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-est....
Grants and funding
- PN-III-P1-1.1-TE-2019-0463/Unitatea Executiva Pentru Finantarea Invatamantului Superior a Cercetarii Dezvoltarii si Inovarii
- Core Programme 21 N/National Research Council
- UIDB/50011/2020/FCT - Fundação para a Ciência e a Tecnologia
- UIDP/50011/2020/FCT - Fundação para a Ciência e a Tecnologia
- LA/P/0006/2020/FCT - Fundação para a Ciência e a Tecnologia
LinkOut - more resources
Full Text Sources

