3D-Printed Polycaprolactone Implants Modified with Bioglass and Zn-Doped Bioglass
- PMID: 36770074
- PMCID: PMC9919585
- DOI: 10.3390/ma16031061
3D-Printed Polycaprolactone Implants Modified with Bioglass and Zn-Doped Bioglass
Abstract
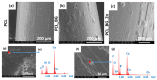
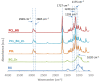
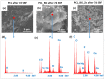
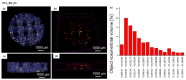
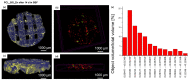
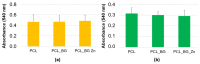
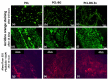
In this work, composite filaments in the form of sticks and 3D-printed scaffolds were investigated as a future component of an osteochondral implant. The first part of the work focused on the development of a filament modified with bioglass (BG) and Zn-doped BG obtained by injection molding. The main outcome was the manufacture of bioactive, strong, and flexible filament sticks of the required length, diameter, and properties. Then, sticks were used for scaffold production. We investigated the effect of bioglass addition on the samples mechanical and biological properties. The samples were analyzed by scanning electron microscopy, optical microscopy, infrared spectroscopy, and microtomography. The effect of bioglass addition on changes in the SBF mineralization process and cell morphology was evaluated. The presence of a spatial microstructure within the scaffolds affects their mechanical properties by reducing them. The tensile strength of the scaffolds compared to filaments was lower by 58-61%. In vitro mineralization experiments showed that apatite formed on scaffolds modified with BG after 7 days of immersion in SBF. Scaffold with Zn-doped BG showed a retarded apatite formation. Innovative 3D-printing filaments containing bioglasses have been successfully applied to print bioactive scaffolds with the surface suitable for cell attachment and proliferation.
Keywords: 3D-printing; bioglass; biomaterials; bone scaffolds; implants; polycaprolactone; zinc.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Nowicki M., Zhu W., Sarkar K., Rao R., Zhang L.G. 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink. Bioprinting. 2020;17:e00066. doi: 10.1016/j.bprint.2019.e00066. - DOI
-
- Gómez-Lizárraga K.K., Flores-Morales C., Del Prado-Audelo M.L., Álvarez-Pérez M.A., Piña-Barba M.C., Escobedo C. Polycaprolactone- and polycaprolactone/ceramic-based 3D-bioplotted porous scaffolds for bone regeneration: A comparative study. Mater. Sci. Eng. C. 2017;79:326–335. doi: 10.1016/j.msec.2017.05.003. - DOI - PubMed
-
- Chiesa-Estomba C.M., Aiastui A., González-Fernández I., Hernáez-Moya R., Rodiño C., Delgado A., Garces J.P., Paredes-Puente J., Aldazabal J., Altuna X., et al. Three-Dimensional Bioprinting Scaffolding for Nasal Cartilage Defects: A Systematic Review. Tissue Eng. Regen. Med. 2021;18:343–353. doi: 10.1007/s13770-021-00331-6. - DOI - PMC - PubMed
-
- Rajzer I., Menaszek E., Bacakova L., Orzelski M., Błażewicz M. Hyaluronic acid-coated carbon nonwoven fabrics as potential material for repair of osteochondral defects. [(accessed on 1 November 2022)];Fibres Text. East. Eur. 2013 99:102–107. Available online: http://www.fibtex.lodz.pl/article937.html.
LinkOut - more resources
Full Text Sources

