Characterization of Dual-Layer Hybrid Biomatrix for Future Use in Cutaneous Wound Healing
- PMID: 36770168
- PMCID: PMC9919111
- DOI: 10.3390/ma16031162
Characterization of Dual-Layer Hybrid Biomatrix for Future Use in Cutaneous Wound Healing
Abstract
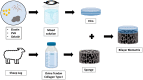
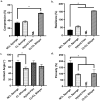
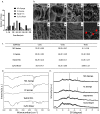
A skin wound without immediate treatment could delay wound healing and may lead to death after severe infection (sepsis). Any interruption or inappropriate normal wound healing, mainly in these wounds, commonly resulted in prolonged and excessive skin contraction. Contraction is a common mechanism in wound healing phases and contributes 40-80% of the original wound size post-healing. Even though it is essential to accelerate wound healing, it also simultaneously limits movement, mainly in the joint area. In the worst-case scenario, prolonged contraction could lead to disfigurement and loss of tissue function. This study aimed to fabricate and characterise the elastin-fortified gelatin/polyvinyl alcohol (PVA) film layered on top of a collagen sponge as a bilayer hybrid biomatrix. Briefly, the combination of halal-based gelatin (4% (w/v)) and PVA ((4% (w/v)) was used to fabricate composite film, followed by the integration of poultry elastin (0.25 mg/mL) and 0.1% (w/v) genipin crosslinking. Furthermore, further analysis was conducted on the composite bilayer biomatrix's physicochemical and mechanical strength. The bilayer biomatrix demonstrated a slow biodegradation rate (0.374967 ± 0.031 mg/h), adequate water absorption (1078.734 ± 42.33%), reasonable water vapour transmission rate (WVTR) (724.6467 ± 70.69 g/m2 h) and porous (102.5944 ± 28.21%). The bilayer biomatrix also exhibited an excellent crosslinking degree and was mechanically robust. Besides, the elastin releasing study presented an acceptable rate post-integration with hybrid biomatrix. Therefore, the ready-to-use bilayer biomatrix will benefit therapeutic effects as an alternative treatment for future diabetic skin wound management.
Keywords: PVA; bilayer scaffold; collagen; elastin; gelatin; wound healing.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Dual-Layered Approach of Ovine Collagen-Gelatin/Cellulose Hybrid Biomatrix Containing Graphene Oxide-Silver Nanoparticles for Cutaneous Wound Healing: Fabrication, Physicochemical, Cytotoxicity and Antibacterial Characterisation.Biomedicines. 2022 Mar 31;10(4):816. doi: 10.3390/biomedicines10040816. Biomedicines. 2022. PMID: 35453566 Free PMC article.
-
Performance of hybrid gelatin-PVA bioinks integrated with genipin through extrusion-based 3D bioprinting: An in vitro evaluation using human dermal fibroblasts.Int J Bioprint. 2023 Feb 7;9(3):677. doi: 10.18063/ijb.677. eCollection 2023. Int J Bioprint. 2023. PMID: 37274005 Free PMC article.
-
Physicochemical Characterization of Bilayer Hybrid Nanocellulose-Collagen as a Potential Wound Dressing.Materials (Basel). 2020 Sep 30;13(19):4352. doi: 10.3390/ma13194352. Materials (Basel). 2020. PMID: 33007893 Free PMC article.
-
Genipin-Crosslinking Effects on Biomatrix Development for Cutaneous Wound Healing: A Concise Review.Front Bioeng Biotechnol. 2022 May 20;10:865014. doi: 10.3389/fbioe.2022.865014. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35677301 Free PMC article. Review.
-
Apigenin Self-Assembled Collagen Biomatrix for Reprogramming the Obese Wound Microenvironment for Its Management and Repair.ACS Appl Bio Mater. 2024 Mar 18;7(3):1317-1335. doi: 10.1021/acsabm.3c00609. Epub 2024 Feb 15. ACS Appl Bio Mater. 2024. PMID: 38357783 Review.
Cited by
-
Plasma-Polymerised Antibacterial Coating of Ovine Tendon Collagen Type I (OTC) Crosslinked with Genipin (GNP) and Dehydrothermal-Crosslinked (DHT) as a Cutaneous Substitute for Wound Healing.Materials (Basel). 2023 Mar 29;16(7):2739. doi: 10.3390/ma16072739. Materials (Basel). 2023. PMID: 37049037 Free PMC article.
-
Biological Safety Assessments of High-Purified Ovine Collagen Type I Biomatrix for Future Therapeutic Product: International Organisation for Standardisation (ISO) and Good Laboratory Practice (GLP) Settings.Polymers (Basel). 2023 May 24;15(11):2436. doi: 10.3390/polym15112436. Polymers (Basel). 2023. PMID: 37299233 Free PMC article.
-
Functionalised Sodium-Carboxymethylcellulose-Collagen Bioactive Bilayer as an Acellular Skin Substitute for Future Use in Diabetic Wound Management: The Evaluation of Physicochemical, Cell Viability, and Antibacterial Effects.Polymers (Basel). 2024 Aug 8;16(16):2252. doi: 10.3390/polym16162252. Polymers (Basel). 2024. PMID: 39204471 Free PMC article.
References
-
- Salerno A., Di Maio E., Iannace S., Netti P.A. Tailoring the pore structure of PCL scaffolds for tissue engineering prepared via gas foaming of multi-phase blends. J. Porous Mater. 2011;19:181–188. doi: 10.1007/s10934-011-9458-9. - DOI
-
- Rey D.F.V., St-Pierre J.P. Handbook of Tissue Engineering Scaffolds: Volume One. Woodhead Publishing; Sawston, UK: 2019. Fabrication techniques of tissue engineering scaffolds; pp. 109–125. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

