Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches
- PMID: 36770218
- PMCID: PMC9921637
- DOI: 10.3390/ma16031212
Freeze Drying of Polymer Nanoparticles and Liposomes Exploiting Different Saccharide-Based Approaches
Abstract
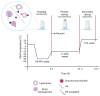
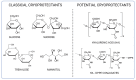
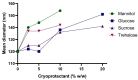
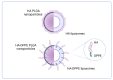
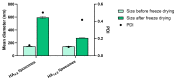
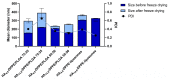
Biodegradable nanocarriers represent promising tools for controlled drug delivery. However, one major drawback related to their use is the long-term stability, which is largely influenced by the presence of water in the formulations, so to solve this problem, freeze-drying with cryoprotectants has been proposed. In the present study, the influence of the freeze-drying procedure on the storage stability of poly(lactide-co-glycolide) (PLGA) nanoparticles and liposomes was evaluated. In particular, conventional cryoprotectants were added to PLGA nanoparticle and liposome formulations in various conditions. Additionally, hyaluronic acid (HA), known for its ability to target the CD44 receptor, was assessed as a cryoprotective excipient: it was added to the nanocarriers as either a free molecule or conjugated to a phospholipid to increase the interaction with the polymer or lipid matrix while exposing HA on the nanocarrier surface. The formulations were resuspended and characterized for size, polydispersity index, zeta potential and morphology. It was demonstrated that only the highest percentages of cryoprotectants allowed the resuspension of stable nanocarriers. Moreover, unlike free HA, HA-phospholipid conjugates were able to maintain the particle mean size after the reconstitution of lyophilized nanoparticles and liposomes. This study paves the way for the use of HA-phospholipids to achieve, at the same time, nanocarrier cryoprotection and active targeting.
Keywords: cryoprotectants; freeze drying; hyaluronic acid; liposomes; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

