High-Pressure Adsorption of CO2 and CH4 on Biochar-A Cost-Effective Sorbent for In Situ Applications
- PMID: 36770272
- PMCID: PMC9920063
- DOI: 10.3390/ma16031266
High-Pressure Adsorption of CO2 and CH4 on Biochar-A Cost-Effective Sorbent for In Situ Applications
Abstract
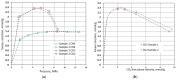
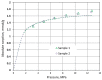
The search for an effective, cost-efficient, and selective sorbent for CO2 capture technologies has been a focus of research in recent years. Many technologies allow efficient separation of CO2 from industrial gases; however, most of them (particularly amine absorption) are very energy-intensive processes not only from the point of view of operation but also solvent production. The aim of this study was to determine CO2 and CH4 sorption capacity of pyrolyzed spruce wood under a wide range of pressures for application as an effective adsorbent for gas separation technology such as Pressure Swing Adsorption (PSA) or Temperature Swing Adsorption (TSA). The idea behind this study was to reduce the carbon footprint related to the transport and manufacturing of sorbent for the separation unit by replacing it with a material that is the direct product of pyrolysis. The results show that pyrolyzed spruce wood has a considerable sorption capacity and selectivity towards CO2 and CH4. Excess sorption capacity reached 1.4 mmol·g-1 for methane and 2.4 mmol·g-1 for carbon dioxide. The calculated absolute sorption capacity was 1.75 mmol·g-1 at 12.6 MPa for methane and 2.7 mmol·g-1 at 4.7 MPa for carbon dioxide. The isotherms follow I type isotherm which is typical for microporous adsorbents.
Keywords: biochar; carbon capture; carbon dioxide; methane; physical adsorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ritchie H., Roser M., Rosado P. CO₂ and Greenhouse Gas Emissions. Our World in Data; Oxford, UK: 2020.
-
- Pörtner H.-O., Roberts D.C., Adams H., Adler C., Aldunce P., Ali E., Begum R.A., Betts R., Kerr R.B., Biesbroek R. Climate Change 2022: Impacts, Adaptation and Vulnerability. IPCC; Geneva, Switzerland: 2022. IPCC Sixth Assessment Report.
-
- Chen S., Liu J., Zhang Q., Teng F., McLellan B.C. A Critical Review on Deployment Planning and Risk Analysis of Carbon Capture, Utilization, and Storage (CCUS) toward Carbon Neutrality. Renew. Sustain. Energy Rev. 2022;167:112537. doi: 10.1016/j.rser.2022.112537. - DOI
-
- de Meyer F., Jouenne S. Industrial Carbon Capture by Absorption: Recent Advances and Path Forward. Curr. Opin. Chem. Eng. 2022;38:100868. doi: 10.1016/j.coche.2022.100868. - DOI
-
- Luis P. Use of Monoethanolamine (MEA) for CO2 Capture in a Global Scenario: Consequences and Alternatives. Desalination. 2016;380:93–99. doi: 10.1016/j.desal.2015.08.004. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

