Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles
- PMID: 36770486
- PMCID: PMC9920192
- DOI: 10.3390/nano13030524
Nanomaterials for Molecular Detection and Analysis of Extracellular Vesicles
Abstract
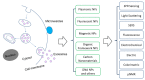
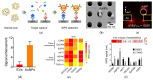
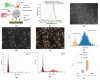
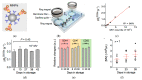
Extracellular vesicles (EVs) have emerged as a novel resource of biomarkers for cancer and certain other diseases. Probing EVs in body fluids has become of major interest in the past decade in the development of a new-generation liquid biopsy for cancer diagnosis and monitoring. However, sensitive and specific molecular detection and analysis are challenging, due to the small size of EVs, low amount of antigens on individual EVs, and the complex biofluid matrix. Nanomaterials have been widely used in the technological development of protein and nucleic acid-based EV detection and analysis, owing to the unique structure and functional properties of materials at the nanometer scale. In this review, we summarize various nanomaterial-based analytical technologies for molecular EV detection and analysis. We discuss these technologies based on the major types of nanomaterials, including plasmonic, fluorescent, magnetic, organic, carbon-based, and certain other nanostructures. For each type of nanomaterial, functional properties are briefly described, followed by the applications of the nanomaterials for EV biomarker detection, profiling, and analysis in terms of detection mechanisms.
Keywords: cancer; diagnostics; exosome; extracellular vesicle; molecular detection; nanomaterials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Fais S., O’Driscoll L., Borras F.E., Buzas E., Camussi G., Cappello F., Carvalho J., Cordeiro da Silva A., Del Portillo H., El Andaloussi S., et al. Evidence-Based Clinical Use of Nanoscale Extracellular Vesicles in Nanomedicine. ACS Nano. 2016;10:3886–3899. doi: 10.1021/acsnano.5b08015. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

