Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel
- PMID: 36770508
- PMCID: PMC9921879
- DOI: 10.3390/nano13030546
Semiconductor Nanomaterial Photocatalysts for Water-Splitting Hydrogen Production: The Holy Grail of Converting Solar Energy to Fuel
Abstract
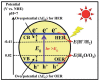
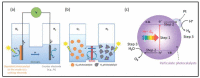
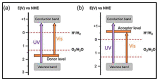
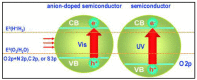
Nanomaterials have attracted attention for application in photocatalytic hydrogen production because of their beneficial properties such as high specific surface area, attractive morphology, and high light absorption. Furthermore, hydrogen is a clean and green source of energy that may help to resolve the existing energy crisis and increasing environmental pollution caused by the consumption of fossil fuels. Among various hydrogen production methods, photocatalytic water splitting is most significant because it utilizes solar light, a freely available energy source throughout the world, activated via semiconductor nanomaterial catalysts. Various types of photocatalysts are developed for this purpose, including carbon-based and transition-metal-based photocatalysts, and each has its advantages and disadvantages. The present review highlights the basic principle of water splitting and various techniques such as the thermochemical process, electrocatalytic process, and direct solar water splitting to enhance hydrogen production. Moreover, modification strategies such as band gap engineering, semiconductor alloys, and multiphoton photocatalysts have been reviewed. Furthermore, the Z- and S-schemes of heterojunction photocatalysts for water splitting were also reviewed. Ultimately, the strategies for developing efficient, practical, highly efficient, and novel visible-light-harvesting photocatalysts will be discussed, in addition to the challenges that are involved. This review can provide researchers with a reference for the current state of affairs, and may motivate them to develop new materials for hydrogen generation.
Keywords: green source; hydrogen production; nanomaterial; photocatalysis; semiconductor materials; water splitting.
Conflict of interest statement
There are no conflict of interest to declare.
Figures




References
-
- Mohsin M., Bhatti I.A., Ashar A., Khan M.W., Farooq M.U., Khan H., Hussain M.T., Loomba S., Mohiuddin M., Zavabeti A. Iron-doped zinc oxide for photocatalyzed degradation of humic acid from municipal wastewater. Appl. Mater. Today. 2021;23:101047. doi: 10.1016/j.apmt.2021.101047. - DOI
-
- Hisatomi T., Domen K. Reaction systems for solar hydrogen production via water splitting with particulate semiconductor photocatalysts. Nat. Catal. 2019;2:387–399. doi: 10.1038/s41929-019-0242-6. - DOI
-
- Tobaldi D., Kočí K., Edelmannová M., Lajaunie L., Figueiredo B., Calvino J., Seabra M., Labrincha J. CuxO and carbon–modified TiO2–based hybrid materials for photocatalytically assisted H2 generation. Mater. Today Energy. 2021;19:100607. doi: 10.1016/j.mtener.2020.100607. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

