Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications
- PMID: 36770578
- PMCID: PMC9921157
- DOI: 10.3390/nano13030618
Polyindole Embedded Nickel/Zinc Oxide Nanocomposites for High-Performance Energy Storage Applications
Abstract
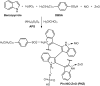
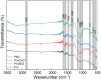
Conducting polymers integrated with metal oxides create opportunities for hybrid capacitive electrodes. In this work, we report a one-pot oxidative polymerization for the synthesis of integrated conductive polyindole/nickel oxide (PIn/NiO), polyindole/zinc oxide (PIn/ZnO), and polyindole/nickel oxide/zinc oxide (PNZ). The polymers were analyzed thoroughly for their composition and physical as well as chemical properties by X-ray diffraction (XRD), scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible spectroscopy (UV-Vis), and thermogravimetric analysis (TGA). The PIn and its composites were processed into electrodes, and their use in symmetrical supercapacitors in two- and three-electrode setups was evaluated by cyclic voltammetry (CV), galvanostatic discharge (GCD), and electrochemical impedance spectroscopy (EIS). The best electrochemical charge storage capability was found for the ternary PNZ composite. The high performance directly correlates with its uniformly shaped nanofibrous structure and high crystallinity. For instance, the symmetrical supercapacitor fabricated with PNZ hybrid electrodes shows a high specific capacitance of 310.9 F g-1 at 0.5 A g-1 with an energy density of 42.1 Wh kg-1, a power density of 13.2 kW kg-1, and a good cycling stability of 78.5% after 5000 cycles. This report presents new electrode materials for advanced supercapacitor technology based on these results.
Keywords: conductive polymers; one-pot synthesis; oxidative polymerization; supercapacitors; ternary hybrid electrodes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kubra K.T., Javaid A., Patil B., Sharif R., Salman A., Shahzadi S., Siddique S., Ghani S. Synthesis and characterization of novel Pr6O11/Mn3O4 nanocomposites for electrochemical supercapacitors. Ceram. Int. 2019;45:6819–6827.
-
- Zhao X., Mao L., Cheng Q., Li J., Liao F., Yang G., Xie L., Zhao C., Chen L. Two-dimensional spinel structured Co-based materials for high performance supercapacitors: A Critical Review. Chem. Eng. J. 2020;387:124081. doi: 10.1016/j.cej.2020.124081. - DOI
-
- Huang C., Hao C., Zheng W., Zhou S., Yang L., Wang X., Zhu L. Synthesis of polyaniline/nickel ox-ide/sulfonated graphene ternary composite for all-solid-state asymmetric supercapacitor. Appl. Surf. Sci. 2020;505:144589. doi: 10.1016/j.apsusc.2019.144589. - DOI
-
- Gao P., Chen Z., Gong Y., Zhang R., Liu H., Tang P., Chen X., Passerini S., Liu J. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: Synthesis, advanced characterization, and fundamentals. Adv. Energy Mater. 2020;10:1903780.
-
- Ramesan M.T., Anjitha T., Parvathi K., Anilkumar T., Mathew G. Nano zinc ferrite filler incorporated polyindole/poly(vinyl alcohol) blend: Preparation, characterization, and investigation of electrical properties. Adv. Polym. Technol. 2018;37:3639–3649. doi: 10.1002/adv.22148. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

