DNA Base Excision Repair Intermediates Influence Duplex-Quadruplex Equilibrium
- PMID: 36770637
- PMCID: PMC9920732
- DOI: 10.3390/molecules28030970
DNA Base Excision Repair Intermediates Influence Duplex-Quadruplex Equilibrium
Abstract
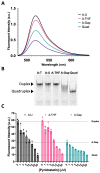
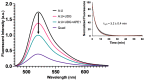
Although genomic DNA is predominantly duplex under physiological conditions, particular sequence motifs can favor the formation of alternative secondary structures, including the G-quadruplex. These structures can exist within gene promoters, telomeric DNA, and regions of the genome frequently found altered in human cancers. DNA is also subject to hydrolytic and oxidative damage, and its local structure can influence the type of damage and its magnitude. Although the repair of endogenous DNA damage by the base excision repair (BER) pathway has been extensively studied in duplex DNA, substantially less is known about repair in non-duplex DNA structures. Therefore, we wanted to better understand the effect of DNA damage and repair on quadruplex structure. We first examined the effect of placing pyrimidine damage products uracil, 5-hydroxymethyluracil, the chemotherapy agent 5-fluorouracil, and an abasic site into the loop region of a 22-base telomeric repeat sequence known to form a G-quadruplex. Quadruplex formation was unaffected by these analogs. However, the activity of the BER enzymes were negatively impacted. Uracil DNA glycosylase (UDG) and single-strand selective monofunctional uracil DNA glycosylase (SMUG1) were inhibited, and apurinic/apyrimidinic endonuclease 1 (APE1) activity was completely blocked. Interestingly, when we performed studies placing DNA repair intermediates into the strand opposite the quadruplex, we found that they destabilized the duplex and promoted quadruplex formation. We propose that while duplex is the preferred configuration, there is kinetic conversion between duplex and quadruplex. This is supported by our studies using a quadruplex stabilizing molecule, pyridostatin, that is able to promote quadruplex formation starting from duplex DNA. Our results suggest how DNA damage and repair intermediates can alter duplex-quadruplex equilibrium.
Keywords: DNA quadruplex; base excision repair; duplex-quadruplex equilibrium; glycosylase; pyridostatin; telomere.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
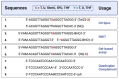
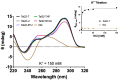
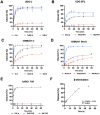
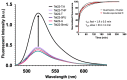
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

